当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemical potential window of battery electrolytes: the HOMO–LUMO misconception†
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2018-07-28 00:00:00 , DOI: 10.1039/c8ee01286e Pekka Peljo 1, 2, 3, 4, 5 , Hubert H. Girault 1, 2, 3, 4, 5
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2018-07-28 00:00:00 , DOI: 10.1039/c8ee01286e Pekka Peljo 1, 2, 3, 4, 5 , Hubert H. Girault 1, 2, 3, 4, 5
Affiliation
|
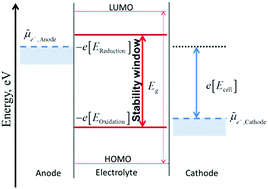
A widespread misconception in the lithium ion battery literature is the equality of the energy difference of HOMO and LUMO of the solvent with the electrochemical stability window. HOMO and LUMO are concepts derived from approximated electronic structure theory while investigating electronic properties of isolated molecules, and their energy levels do not indicate species participating in redox reactions. On the other hand, redox potentials are directly related to the Gibbs free energy difference of the reactants and products. While redox potentials in some cases show strong correlation with HOMO energies, the offset can be of several eVs. Presence of electrolytes and other molecules can also significantly affect the redox potentials of the solvent leading to offset as high as 4 eV from the HOMO energies. In this opinion we provide a correct thermodynamic representation for the electrochemical stability of the electrolyte, based on redox potentials and Fermi level of the electron in solution, and demonstrate that the use of terms HOMO and LUMO should be avoided when talking about the electrochemical stability of electrolytes. Instead, it is more correct to speak of potential of electrolyte reduction at negative potentials, and of potential of solvent oxidation at positive potentials.
中文翻译:

电池电解质的电化学势能窗口:HOMO-LUMO误解†
锂离子电池文献中普遍存在的误解是溶剂的HOMO和LUMO的能量差与电化学稳定性窗口相等。HOMO和LUMO是从近似电子结构理论衍生而来的,同时研究了孤立分子的电子性质,它们的能级并不表明物种参与了氧化还原反应。另一方面,氧化还原电势与反应物和产物的吉布斯自由能差直接相关。尽管在某些情况下氧化还原电势与HOMO能量有很强的相关性,但偏移量可能为几个eV。电解质和其他分子的存在也会显着影响溶剂的氧化还原电势,从而导致HOMO能量偏移高达4 eV。在这种观点下,我们基于氧化还原电势和溶液中电子的费米能级提供了电解质电化学稳定性的正确热力学表示,并证明了在谈论电解质的电化学稳定性时应避免使用术语HOMO和LUMO。电解质。相反,在负电势下说电解质还原电势,在正电势下说溶剂氧化电势更正确。
更新日期:2018-07-28
中文翻译:

电池电解质的电化学势能窗口:HOMO-LUMO误解†
锂离子电池文献中普遍存在的误解是溶剂的HOMO和LUMO的能量差与电化学稳定性窗口相等。HOMO和LUMO是从近似电子结构理论衍生而来的,同时研究了孤立分子的电子性质,它们的能级并不表明物种参与了氧化还原反应。另一方面,氧化还原电势与反应物和产物的吉布斯自由能差直接相关。尽管在某些情况下氧化还原电势与HOMO能量有很强的相关性,但偏移量可能为几个eV。电解质和其他分子的存在也会显着影响溶剂的氧化还原电势,从而导致HOMO能量偏移高达4 eV。在这种观点下,我们基于氧化还原电势和溶液中电子的费米能级提供了电解质电化学稳定性的正确热力学表示,并证明了在谈论电解质的电化学稳定性时应避免使用术语HOMO和LUMO。电解质。相反,在负电势下说电解质还原电势,在正电势下说溶剂氧化电势更正确。



























 京公网安备 11010802027423号
京公网安备 11010802027423号