当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gold(I)‐catalyzed Benzylation of (Hetero)aryl Boronic Acids with (Hetero)benzyl Bromides by the Strategy of a SN2‐type Reaction
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2018-08-31 , DOI: 10.1002/asia.201800923 Wenqing Zang 1 , Yin Wei 1 , Min Shi 1
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2018-08-31 , DOI: 10.1002/asia.201800923 Wenqing Zang 1 , Yin Wei 1 , Min Shi 1
Affiliation
|
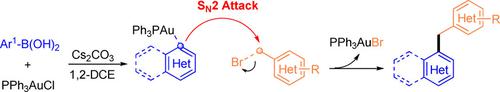
Herein, the first example of gold‐catalyzed benzylation of (hetero)aryl boronic acids with (hetero)benzyl bromides to give the corresponding cross‐coupling products in moderate to good yields is reported. The reaction proceeds through a possible intermolecular SN2‐type reaction pathway to give a wide variety of di(hetero)arylmethanes as the desired products. An intriguing reaction mechanism has been proposed on the basis of control experiments, 31P‐NMR spectroscopic detection and DFT calculations.
中文翻译:

金(I)催化的(杂)苄基溴与(杂)苄基溴的SN2型反应策略
在此,报道了第一个用(杂)苄基溴对(杂)芳基硼酸进行金催化的苄基化反应,以得到中等至良好收率的相应交叉偶联产物的实例。反应通过可能的分子间S N 2型反应路径进行,从而得到各种所需的二(杂)芳基甲烷。在控制实验,31 P-NMR光谱检测和DFT计算的基础上,提出了一种有趣的反应机理。
更新日期:2018-08-31
中文翻译:

金(I)催化的(杂)苄基溴与(杂)苄基溴的SN2型反应策略
在此,报道了第一个用(杂)苄基溴对(杂)芳基硼酸进行金催化的苄基化反应,以得到中等至良好收率的相应交叉偶联产物的实例。反应通过可能的分子间S N 2型反应路径进行,从而得到各种所需的二(杂)芳基甲烷。在控制实验,31 P-NMR光谱检测和DFT计算的基础上,提出了一种有趣的反应机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号