当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conformational Conversion during Controlled Oligomerization into Nonamylogenic Protein Nanoparticles
Biomacromolecules ( IF 5.5 ) Pub Date : 2018-07-27 00:00:00 , DOI: 10.1021/acs.biomac.8b00924 Julieta M. Sánchez 1, 2 , Laura Sánchez-García 3, 4 , Mireia Pesarrodona 1, 3, 4 , Naroa Serna 1, 3, 4 , Alejandro Sánchez-Chardi 5 , Ugutz Unzueta 1, 3, 4, 6 , Ramón Mangues 4, 6 , Esther Vázquez 1, 3, 4 , Antonio Villaverde 1, 3, 4
Biomacromolecules ( IF 5.5 ) Pub Date : 2018-07-27 00:00:00 , DOI: 10.1021/acs.biomac.8b00924 Julieta M. Sánchez 1, 2 , Laura Sánchez-García 3, 4 , Mireia Pesarrodona 1, 3, 4 , Naroa Serna 1, 3, 4 , Alejandro Sánchez-Chardi 5 , Ugutz Unzueta 1, 3, 4, 6 , Ramón Mangues 4, 6 , Esther Vázquez 1, 3, 4 , Antonio Villaverde 1, 3, 4
Affiliation
|
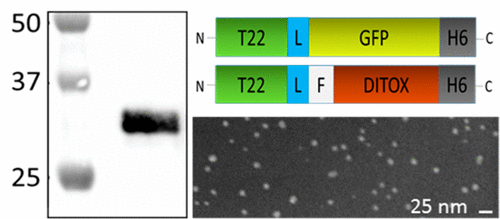
Protein materials are rapidly gaining interest in materials sciences and nanomedicine because of their intrinsic biocompatibility and full biodegradability. The controlled construction of supramolecular entities relies on the controlled oligomerization of individual polypeptides, achievable through different strategies. Because of the potential toxicity of amyloids, those based on alternative molecular organizations are particularly appealing, but the structural bases on nonamylogenic oligomerization remain poorly studied. We have applied spectrofluorimetry and spectropolarimetry to identify the conformational conversion during the oligomerization of His-tagged cationic stretches into regular nanoparticles ranging around 11 nm, useful for tumor-targeted drug delivery. We demonstrate that the novel conformation acquired by the proteins, as building blocks of these supramolecular assemblies, shows different extents of compactness and results in a beta structure enrichment that enhances their structural stability. The conformational profiling presented here offers clear clues for understanding and tailoring the process of nanoparticle formation through the use of cationic and histidine rich stretches in the context of protein materials usable in advanced nanomedical strategies.
中文翻译:

受控低聚反应过程中的构象转化为非淀粉生成蛋白纳米粒子
蛋白质材料因其固有的生物相容性和完全的生物降解性而在材料科学和纳米医学中迅速引起人们的兴趣。超分子实体的受控构建依赖于通过不同策略可实现的单个多肽的受控寡聚。由于淀粉样蛋白具有潜在的毒性,基于替代分子组织的淀粉样蛋白特别有吸引力,但基于非淀粉形成的低聚的结构基础仍然研究不足。我们已经应用了光谱荧光法和光谱极化法来鉴定在His标记的阳离子片段寡聚化成约11 nm左右的规则纳米颗粒过程中的构象转化,可用于靶向肿瘤的药物递送。我们证明了蛋白质获得的新颖构象,作为这些超分子组装的组成部分,它们显示出不同程度的致密性,并导致β结构富集,从而增强了其结构稳定性。本文介绍的构象分析为在先进的纳米医学策略中使用的蛋白质材料的背景下,通过使用富含阳离子和组氨酸的链段,为理解和定制纳米颗粒的形成过程提供了清晰的线索。
更新日期:2018-07-27
中文翻译:

受控低聚反应过程中的构象转化为非淀粉生成蛋白纳米粒子
蛋白质材料因其固有的生物相容性和完全的生物降解性而在材料科学和纳米医学中迅速引起人们的兴趣。超分子实体的受控构建依赖于通过不同策略可实现的单个多肽的受控寡聚。由于淀粉样蛋白具有潜在的毒性,基于替代分子组织的淀粉样蛋白特别有吸引力,但基于非淀粉形成的低聚的结构基础仍然研究不足。我们已经应用了光谱荧光法和光谱极化法来鉴定在His标记的阳离子片段寡聚化成约11 nm左右的规则纳米颗粒过程中的构象转化,可用于靶向肿瘤的药物递送。我们证明了蛋白质获得的新颖构象,作为这些超分子组装的组成部分,它们显示出不同程度的致密性,并导致β结构富集,从而增强了其结构稳定性。本文介绍的构象分析为在先进的纳米医学策略中使用的蛋白质材料的背景下,通过使用富含阳离子和组氨酸的链段,为理解和定制纳米颗粒的形成过程提供了清晰的线索。











































 京公网安备 11010802027423号
京公网安备 11010802027423号