Chemical Physics Letters ( IF 2.8 ) Pub Date : 2018-07-26 , DOI: 10.1016/j.cplett.2018.07.058 Nicolay N. Golovnev , Maxim S. Molokeev , Irina V. Sterkhova , Maxim K. Lesnikov
|
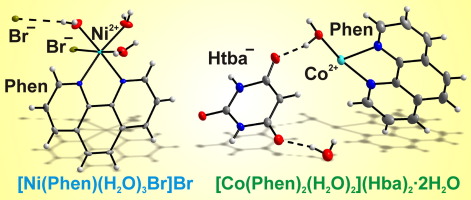
Two nickel(II) and cobalt(II) complexes with phenanthroline, [Ni(Phen)(H2O)3Br]Br (1) and [Co(Phen)2(H2O)2](Hba)2∙2H2O (2), Phen = 1,10-phenanthroline and Hba− = barbiturate anion, were synthesized and characterized by powder XRD, TGA and FT-IR. Their structures were determined by single crystal X-ray diffraction techniques. The Ni2+ ion is coordinated by two N atoms of Phen molecule, Br− ion and three H2O molecules forming an octahedron. Uncoordinated and coordinated Br− ions are connected with water molecules by O—H∙∙∙Br intermolecular hydrogen bonds with the formation of a 2D plane network which is extended into a 3D network by π−π stacking interactions. The [Co(Phen)2(H2O)2]2+ cation contains a six-coordinated cobalt atom chelated by two Phen ligands and two aqua ligands in the cis arrangement. N—H∙∙∙O, O—H∙∙∙O and C—H∙∙∙O intermolecular hydrogen bonds form a 3D net. N—H∙∙∙O hydrogen bonds form the infinite chains of Hba–. In addition, coordinated Phen molecules and lattice water molecules are linked via C—H∙∙∙OW hydrogen bonds to form infinite zigzag chains. These different chains are connected by OW—H∙∙∙O hydrogen bonds. π−π interaction plays an important role in the stabilization of structures 1-2. FT-IR, TGA, the diffuse reflectance and UV-Vis spectra were also used to characterize these compounds.
中文翻译:

带有1,10-菲咯啉的两种新型混合配体Ni(II)和Co(II)配合物:合成,结构表征和热稳定性
两种与菲咯啉,[Ni(Phen)(H 2 O)3 Br] Br(1)和[Co(Phen)2(H 2 O)2 ] [Hba)2 ∙邻位的钴(II)配合物2H 2 O(2),Phen的= 1,10-菲咯啉和HBA - =巴比妥阴离子,被合成和表征通过粉末XRD,TGA和FT-IR。它们的结构通过单晶X射线衍射技术确定。将Ni 2+离子通过Phen的分子,Br与两个N原子配位-离子和三个H 2水分子形成一个八面体。不协调,协调溴-离子通过O-H∙∙∙Br分子间氢键与水分子连接,形成2D平面网络,并通过π-π堆积相互作用扩展为3D网络。[Co(Phen)2(H 2 O)2 ] 2+阳离子含有被两个Phen配体和两个aqua配体以顺式螯合的六配位钴原子。N-H∙∙∙O,OH-H∙∙∙O和C-H∙∙∙O分子间氢键形成3D网络。N-H∙∙∙O氢键形成Hba –的无限链。此外,配位的Phen分子和晶格水分子通过C-H∙∙∙OW氢键形成无限的锯齿形链。这些不同的链通过OW-H∙∙∙O氢键连接。π-π相互作用在结构的稳定化中起重要作用1 - 2。FT-IR,TGA,漫反射率和UV-Vis光谱也用于表征这些化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号