Chem ( IF 23.5 ) Pub Date : 2018-07-26 , DOI: 10.1016/j.chempr.2018.07.003 Pan Gao , Chengkai Yuan , Yue Zhao , Zhuangzhi Shi
|
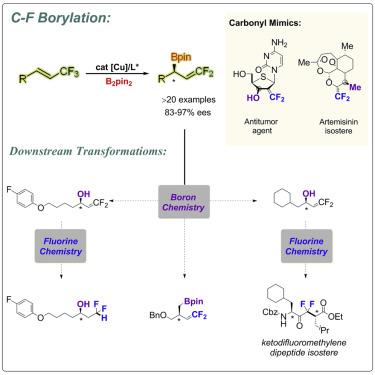
gem-Difluoroalkenes have steric and electronic profiles similar to those of ketones, aldehydes, and esters, and consequently have been used widely as carbonyl isosteres in modern drug discovery. Although many attempts have been made to achieve gem-difluoroalkenes, the induction of enantioselectivity at the α position of a gem-difluorovinyl group still remains a challenge. Herein, an efficient method for the construction of gem-difluoroallylboronates with high enantiomeric excess via a copper-catalyzed defluoroborylation of 1-(trifluoromethyl)alkenes with B2pin2 is described. The reaction conditions were mild, and a variety of common functional groups, such as ether, fluoride, chloride, bromide, iodide, ester, cyano, sulfide, amino, and indoyl groups, were well tolerated. Furthermore, we not only applied this developed system as a powerful synthetic tool for the late-stage modification of complex compounds but also highlighted the utility of the formed compounds in synthesis.
中文翻译:

铜催化的1-(三氟甲基)烯烃的不对称脱氟硼化
宝石-二氟烯烃具有类似于酮,醛和酯的立体和电子构型,因此在现代药物发现中已广泛用作羰基等排体。尽管已经进行了许多尝试来获得宝石-二氟烯烃,但是在宝石-二氟乙烯基的α位上诱导对映选择性仍然是一个挑战。这里,为了建造一种有效的方法宝石经由1-(三氟甲基)烯烃的铜-催化的defluoroborylation具有高对映体过量-difluoroallylboronates与乙2销2描述。反应条件温和,对各种常见的官能团(如醚,氟,氯,溴,碘,酯,氰基,硫化物,氨基和吲哚基)具有良好的耐受性。此外,我们不仅将此开发的系统用作复杂化合物后期修饰的强大合成工具,还强调了所形成化合物在合成中的实用性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号