当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enhancement of electrocatalytic abilities for reducing carbon dioxide: functionalization with a redox-active ligand-coordinated metal complex†
Dalton Transactions ( IF 4 ) Pub Date : 2018-07-25 00:00:00 , DOI: 10.1039/c8dt02288g Habib Md. Ahsan 1, 2, 3, 4, 5 , Brian K. Breedlove 1, 2, 3, 4, 5 , Santivongskul Piangrawee 1, 2, 3, 4, 5 , Mohammad Rasel Mian 1, 2, 3, 4, 5 , Ahmed Fetoh 1, 6, 7, 8, 9 , Goulven Cosquer 1, 2, 3, 4, 5 , Masahiro Yamashita 1, 2, 3, 4, 5
Dalton Transactions ( IF 4 ) Pub Date : 2018-07-25 00:00:00 , DOI: 10.1039/c8dt02288g Habib Md. Ahsan 1, 2, 3, 4, 5 , Brian K. Breedlove 1, 2, 3, 4, 5 , Santivongskul Piangrawee 1, 2, 3, 4, 5 , Mohammad Rasel Mian 1, 2, 3, 4, 5 , Ahmed Fetoh 1, 6, 7, 8, 9 , Goulven Cosquer 1, 2, 3, 4, 5 , Masahiro Yamashita 1, 2, 3, 4, 5
Affiliation
|
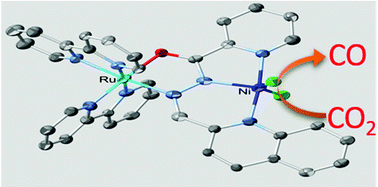
A binary system consisting of a ditopic planar pseudo-pincer ligand (qlca = quinoline-2-carbaldehyde (pyridine-2-carbonyl) hydrazone) coordinated to two metal centres affording [{Ru(bpy)2}(μ-qlca)NiCl2]Cl·4H2O·CH3OH (2) (bpy = 2,2′-bipyridine) is reported. The Ni2+ moiety acts as the electrocatalytic active site for CO2 reduction to CO. The turnover frequency (TOF) increased from 0.83 s−1 for [Ni(qlca)Cl2] (3) to 120 s−1 for 2, and the overpotential is 350 mV less than that for 3 due to the electronic influence of the {Ru(bpy)2}2+ moiety on the catalytic active site.
中文翻译:

增强减少二氧化碳的电催化能力:氧化还原活性配体配位的金属络合物的功能化†
由二位平面伪钳位配体(qlca =喹啉-2-甲醛(吡啶-2-羰基))与两个金属中心配位的二元体系,提供[{Ru(bpy)2 }(μ-qlca)NiCl 2据报道有] Cl·4H 2 O·CH 3 OH(2)(bpy = 2,2'-联吡啶)。在Ni 2+部分充当电催化活性位点的CO 2还原成CO。的转换频率(TOF)从0.83 s增加到-1为[镍(qlca)氯2 ](3),以120秒-1为2,且超电势比3的电势低350 mV由于{Ru(bpy)2 } 2+部分对催化活性位点的电子影响。
更新日期:2018-07-25
中文翻译:

增强减少二氧化碳的电催化能力:氧化还原活性配体配位的金属络合物的功能化†
由二位平面伪钳位配体(qlca =喹啉-2-甲醛(吡啶-2-羰基))与两个金属中心配位的二元体系,提供[{Ru(bpy)2 }(μ-qlca)NiCl 2据报道有] Cl·4H 2 O·CH 3 OH(2)(bpy = 2,2'-联吡啶)。在Ni 2+部分充当电催化活性位点的CO 2还原成CO。的转换频率(TOF)从0.83 s增加到-1为[镍(qlca)氯2 ](3),以120秒-1为2,且超电势比3的电势低350 mV由于{Ru(bpy)2 } 2+部分对催化活性位点的电子影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号