当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adaptive responses of sterically confined intramolecular chalcogen bonds†
Chemical Science ( IF 7.6 ) Pub Date : 2018-07-25 00:00:00 , DOI: 10.1039/c8sc01943f Karuthapandi Selvakumar 1 , Harkesh B Singh 2
Chemical Science ( IF 7.6 ) Pub Date : 2018-07-25 00:00:00 , DOI: 10.1039/c8sc01943f Karuthapandi Selvakumar 1 , Harkesh B Singh 2
Affiliation
|
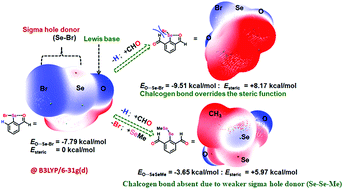
The responsive behavior of an entity towards its immediate surrounding is referred to as an adaptive response. The adaptive responses of a noncovalent interaction at the molecular scale are reflected from its structural and functional roles. Intramolecular chalcogen bonding (IChB), an attractive interaction between a heavy chalcogen E (E = Se or Te) centered sigma hole and an ortho-heteroatom Lewis base donor D (D = O or N), plays an adaptive role in defining the structure and reactivity of arylchalcogen compounds. In this perspective, we describe the adaptive roles of a chalcogen centered Lewis acid sigma hole and a proximal Lewis base (O or N) in accommodating built-in steric stress in 2,6-disubstituted arylchalcogen compounds. From our perspective, the IChB components (a sigma hole and the proximal Lewis base) act in synergism to accommodate the overwhelming steric force. The adaptive responses of the IChB components are inferred from the observed molecular structures and reactivity. These include (a) adaptation of a conformation without IChBs, (b) adaptation of a conformation with weak IChBs, (c) twisting the skeletal aryl ring while maintaining IChBs, (d) ionization of the E–X bond (e.g., X = Br) to relieve stress and (e) intramolecular cyclization to relieve steric stress. A comprehensive approach, involving X-ray data analysis, density functional theory (DFT) calculations, reaction pattern analysis and principal component analysis (PCA), has been employed to rationalize the adaptive behaviors of IChBs in arylchalcogen compounds. We believe that the perception of ChB as an adaptive/stimulus responsive interaction would profit the futuristic approaches that would utilise ChB as self-assembly and molecular recognition tools.
中文翻译:

空间受限分子内硫属元素键的自适应响应†
实体对其周围环境的响应行为称为自适应响应。分子尺度上非共价相互作用的适应性反应反映在其结构和功能作用上。分子内硫属元素键合 (IChB),重硫属元素 E(E = Se 或 Te)中心 sigma 孔与邻位之间的有吸引力的相互作用-杂原子路易斯碱基供体 D(D = O 或 N),在定义芳基硫属化合物的结构和反应性方面发挥着适应性作用。从这个角度来看,我们描述了以硫属元素为中心的路易斯酸 σ 空穴和近端路易斯碱(O 或 N)在适应 2,6-二取代芳基硫属化合物中的内置空间应力中的适应性作用。从我们的角度来看,IChB 组件(sigma 孔和近端 Lewis 碱基)协同作用以适应压倒性的空间力。从观察到的分子结构和反应性推断 IChB 组分的适应性反应。这些包括 (a) 适应没有 IChBs 的构象,(b) 适应具有弱 IChBs 的构象,(c) 在保持 IChBs 的同时扭曲骨架芳基环,(d) E-X 键的电离(例如, X = Br) 以减轻应力和 (e) 分子内环化以减轻空间应力。一种综合方法,包括 X 射线数据分析、密度泛函理论 (DFT) 计算、反应模式分析和主成分分析 (PCA),已被用于合理化 IChB 在芳基硫属化合物中的适应性行为。我们相信,将 ChB 视为一种适应性/刺激响应性相互作用,将有利于利用 ChB 作为自组装和分子识别工具的未来方法。
更新日期:2018-07-25
中文翻译:

空间受限分子内硫属元素键的自适应响应†
实体对其周围环境的响应行为称为自适应响应。分子尺度上非共价相互作用的适应性反应反映在其结构和功能作用上。分子内硫属元素键合 (IChB),重硫属元素 E(E = Se 或 Te)中心 sigma 孔与邻位之间的有吸引力的相互作用-杂原子路易斯碱基供体 D(D = O 或 N),在定义芳基硫属化合物的结构和反应性方面发挥着适应性作用。从这个角度来看,我们描述了以硫属元素为中心的路易斯酸 σ 空穴和近端路易斯碱(O 或 N)在适应 2,6-二取代芳基硫属化合物中的内置空间应力中的适应性作用。从我们的角度来看,IChB 组件(sigma 孔和近端 Lewis 碱基)协同作用以适应压倒性的空间力。从观察到的分子结构和反应性推断 IChB 组分的适应性反应。这些包括 (a) 适应没有 IChBs 的构象,(b) 适应具有弱 IChBs 的构象,(c) 在保持 IChBs 的同时扭曲骨架芳基环,(d) E-X 键的电离(例如, X = Br) 以减轻应力和 (e) 分子内环化以减轻空间应力。一种综合方法,包括 X 射线数据分析、密度泛函理论 (DFT) 计算、反应模式分析和主成分分析 (PCA),已被用于合理化 IChB 在芳基硫属化合物中的适应性行为。我们相信,将 ChB 视为一种适应性/刺激响应性相互作用,将有利于利用 ChB 作为自组装和分子识别工具的未来方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号