当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
tert‐Butyl Nitrite Mediated Different Functionalizations of Internal Alkenes: Paths to Furoxans and Nitroalkenes
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-08-14 , DOI: 10.1002/adsc.201800668 Bilal Ahmad Mir 1 , Sarangthem Joychandra Singh 1 , Ritush Kumar 1 , Bhisma K. Patel 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-08-14 , DOI: 10.1002/adsc.201800668 Bilal Ahmad Mir 1 , Sarangthem Joychandra Singh 1 , Ritush Kumar 1 , Bhisma K. Patel 1
Affiliation
|
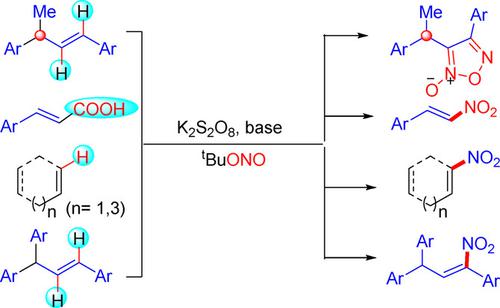
tert‐Butyl nitrite (TBN) reacts differently with various internal alkenes leading to interesting and useful products. Synthesis of 1,2,5‐oxadiazole‐N‐oxides (furoxans) has been achieved from internal alkenes using tert‐butyl nitrite (TBN), quinoline and K2S2O8. Under an identical reaction condition α,β‐unsaturated carboxylic acids and cyclic and acyclic internal alkenes both afforded nitroalkenes as the sole product via decarboxylative and direct nitration path respectively.
中文翻译:

亚硝酸叔丁酯介导内部烯烃的不同功能化:呋喃烷和硝基烯烃的途径
亚硝酸叔丁酯(TBN)与各种内部烯烃的反应不同,从而产生有趣且有用的产物。使用亚硝酸叔丁酯(TBN),喹啉和K 2 S 2 O 8可从内部烯烃中合成1,2,5-恶二唑N-氧化物(呋喃烷)。在相同的反应条件下,α,β-不饱和羧酸以及环状和无环内部烯烃都分别通过脱羧和直接硝化途径提供了硝基烯烃作为唯一产物。
更新日期:2018-08-14
中文翻译:

亚硝酸叔丁酯介导内部烯烃的不同功能化:呋喃烷和硝基烯烃的途径
亚硝酸叔丁酯(TBN)与各种内部烯烃的反应不同,从而产生有趣且有用的产物。使用亚硝酸叔丁酯(TBN),喹啉和K 2 S 2 O 8可从内部烯烃中合成1,2,5-恶二唑N-氧化物(呋喃烷)。在相同的反应条件下,α,β-不饱和羧酸以及环状和无环内部烯烃都分别通过脱羧和直接硝化途径提供了硝基烯烃作为唯一产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号