当前位置:
X-MOL 学术
›
Photochem. Photobiol. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photophysical properties of diethylhexyl 2,6-naphthalate (Corapan TQ), a photostabilizer for sunscreens†
Photochemical & Photobiological Sciences ( IF 3.1 ) Pub Date : 2018-07-20 00:00:00 , DOI: 10.1039/c8pp00204e Ryohei Shimizu 1, 2, 3, 4, 5 , Mikio Yagi 1, 2, 3, 4, 5 , Nozomi Oguchi-Fujiyama 5, 6, 7 , Kazuyuki Miyazawa 5, 6, 7 , Azusa Kikuchi 1, 2, 3, 4, 5
Photochemical & Photobiological Sciences ( IF 3.1 ) Pub Date : 2018-07-20 00:00:00 , DOI: 10.1039/c8pp00204e Ryohei Shimizu 1, 2, 3, 4, 5 , Mikio Yagi 1, 2, 3, 4, 5 , Nozomi Oguchi-Fujiyama 5, 6, 7 , Kazuyuki Miyazawa 5, 6, 7 , Azusa Kikuchi 1, 2, 3, 4, 5
Affiliation
|
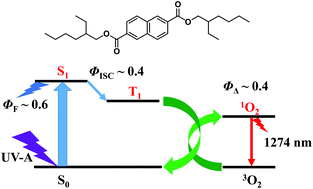
Diethylhexyl 2,6-naphthalate (DEHN, tradename Corapan TQ) is used as a photostabilizer for a widely-used UV-A absorber, butylmethoxydibenzoylmethane (BMDBM). The photophysical properties of DEHN have been studied through measurements of the transient absorption, fluorescence, phosphorescence and EPR spectra in ethanol. The energy level of the lowest excited singlet state of DEHN is higher than that of BMDBM (enol form). The singlet–singlet energy transfer from BMDBM to DEHN is energetically unfavourable. The energy level of the lowest excited triplet (T1) state of DEHN is lower than that of BMDBM. It is probable that the mechanism of photostabilization of BMDBM by DEHN is the triplet–triplet energy transfer from BMDBM to DEHN. The observed phosphorescence spectrum, T1 lifetime and zero-field splitting parameters indicate that the T1 state of DEHN can be regarded mainly as a locally excited 3ππ* state within naphthalene. The sum of the quantum yields of fluorescence of DEHN (0.59 ± 0.09) and singlet oxygen generation by DEHN (0.44 ± 0.04) is almost unity in ethanol. About 60% of the UV energy absorbed by DEHN is released as fluorescence, while about 40% is used to produce the excited triplet state. The photoexcited states of 2-naphthoic acid have been studied for comparison.
中文翻译:

2,6-萘二甲酸二乙基己酯(Corapan TQ)的光物理性质,用于防晒的光稳定剂†
2,6-萘二甲酸二乙基己酯(DEHN,商品名Corapan TQ)用作广泛使用的UV-A吸收剂丁基甲氧基二苯甲酰甲烷(BMDBM)的光稳定剂。通过测量乙醇中的瞬态吸收,荧光,磷光和EPR光谱,研究了DEHN的光物理性质。DEHN的最低激发单重态的能级高于BMDBM(烯醇形式)的能级。从BMDBM到DEHN的单重态-单态能量转移在能量上不利。DEHN的最低激发三重态(T 1)态的能级低于BMDBM的能级。DEHN使BMDBM光稳定的机理很可能是从BMDBM到DEHN的三重态-三重态能量转移。观察到的磷光光谱,T 1寿命和零场分裂参数指示的T 1 DEHN的状态可以被认为主要是作为局部兴奋3萘内ππ*状态。在乙醇中,DEHN的荧光的量子产率(0.59±0.09)和DEHN产生的单线态氧的总量子产率(0.44±0.04)之和。DEHN吸收的大约60%的紫外线能量以荧光形式释放,而大约40%的能量用于产生激发三重态。为了比较,已经研究了2-萘甲酸的光激发态。
更新日期:2018-07-20
中文翻译:

2,6-萘二甲酸二乙基己酯(Corapan TQ)的光物理性质,用于防晒的光稳定剂†
2,6-萘二甲酸二乙基己酯(DEHN,商品名Corapan TQ)用作广泛使用的UV-A吸收剂丁基甲氧基二苯甲酰甲烷(BMDBM)的光稳定剂。通过测量乙醇中的瞬态吸收,荧光,磷光和EPR光谱,研究了DEHN的光物理性质。DEHN的最低激发单重态的能级高于BMDBM(烯醇形式)的能级。从BMDBM到DEHN的单重态-单态能量转移在能量上不利。DEHN的最低激发三重态(T 1)态的能级低于BMDBM的能级。DEHN使BMDBM光稳定的机理很可能是从BMDBM到DEHN的三重态-三重态能量转移。观察到的磷光光谱,T 1寿命和零场分裂参数指示的T 1 DEHN的状态可以被认为主要是作为局部兴奋3萘内ππ*状态。在乙醇中,DEHN的荧光的量子产率(0.59±0.09)和DEHN产生的单线态氧的总量子产率(0.44±0.04)之和。DEHN吸收的大约60%的紫外线能量以荧光形式释放,而大约40%的能量用于产生激发三重态。为了比较,已经研究了2-萘甲酸的光激发态。



























 京公网安备 11010802027423号
京公网安备 11010802027423号