Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Suzuki–Miyaura reaction as a tool for modification of phenoxyl-nitroxyl radicals of the 4H-imidazole N-oxide series†
RSC Advances ( IF 3.9 ) Pub Date : 2018-07-20 00:00:00 , DOI: 10.1039/c8ra05103h Yury A Ten 1 , Oleg G Salnikov 2 , Svetlana A Amitina 1 , Dmitri V Stass 2, 3 , Tatyana V Rybalova 1, 2 , Maxim S Kazantsev 1, 2 , Artem S Bogomyakov 4 , Evgeny A Mostovich 1, 2 , Dmitrii G Mazhukin 1, 2
RSC Advances ( IF 3.9 ) Pub Date : 2018-07-20 00:00:00 , DOI: 10.1039/c8ra05103h Yury A Ten 1 , Oleg G Salnikov 2 , Svetlana A Amitina 1 , Dmitri V Stass 2, 3 , Tatyana V Rybalova 1, 2 , Maxim S Kazantsev 1, 2 , Artem S Bogomyakov 4 , Evgeny A Mostovich 1, 2 , Dmitrii G Mazhukin 1, 2
Affiliation
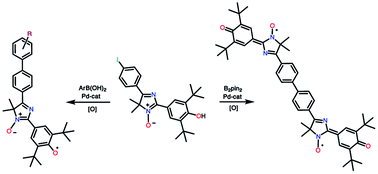
|
2-(3,5-Di-tert-butyl-4-hydroxyphenyl)-5-(4-iodophenyl)-4,4-dimethyl-4H-imidazole 3-oxide reacts with phenylboronic acid and its substituted derivatives in a cross-coupling reaction of the Suzuki–Miyaura type to form 5-biphenyl derivatives of 4H-imidazole-N-oxide. Interaction of the same compound with B2(pin)2 in the presence of PdCl2(PPh3)2 proceeds through the formation of intermediate 1,3,2-dioxoborolane and leads to the product of homocoupling: biphenyl-bis(imidazole). Oxidation of the resultant imidazoles with lead dioxide quantitatively yields stable conjugated phenoxyl-nitroxyl mono- and diradicals, which are of interest as electroactive paramagnetic materials. The crystal structure of the monoradical, 2,6-di-tert-butyl-4-[1-oxido-4-(biphenyl-4-yl)-5,5-dimethyl-1H-imidazole-2(5H)-ylidene]cyclohex-2,5-dienone, its magnetic susceptibility, EPR spectra of the obtained hybrid radicals in solution, and cyclic voltammetry characteristics of 4H-imidazoles were studied.
中文翻译:

铃木-宫浦反应作为修饰 4H-咪唑 N-氧化物系列苯氧基-硝酰基自由基的工具†
2-(3,5-二叔丁基-4-羟基苯基)-5-(4-碘苯基)-4,4-二甲基-4H-咪唑3-氧化物与苯基硼酸及其取代衍生物发生交叉反应-铃木-宫浦型偶联反应,形成 4 H-咪唑-N-氧化物的 5-联苯衍生物。在 PdCl 2 (PPh 3 ) 2存在下,同一化合物与 B 2 (pin) 2相互作用,形成中间体 1,3,2-二氧硼杂硼烷,并产生自偶联产物:联苯-双(咪唑) 。用二氧化铅氧化所得咪唑定量产生稳定的共轭苯氧基-硝酰基单基和双基,它们作为电活性顺磁材料而受到关注。单自由基 2,6-二叔丁基-4-[1-氧化-4-(联苯-4-基)-5,5-二甲基-1 H -咪唑-2(5 H ) 的晶体结构研究了[亚基]环己-2,5-二烯酮及其磁化率、所得杂化自由基在溶液中的EPR谱以及4 H-咪唑的循环伏安特性。
更新日期:2018-07-20
中文翻译:

铃木-宫浦反应作为修饰 4H-咪唑 N-氧化物系列苯氧基-硝酰基自由基的工具†
2-(3,5-二叔丁基-4-羟基苯基)-5-(4-碘苯基)-4,4-二甲基-4H-咪唑3-氧化物与苯基硼酸及其取代衍生物发生交叉反应-铃木-宫浦型偶联反应,形成 4 H-咪唑-N-氧化物的 5-联苯衍生物。在 PdCl 2 (PPh 3 ) 2存在下,同一化合物与 B 2 (pin) 2相互作用,形成中间体 1,3,2-二氧硼杂硼烷,并产生自偶联产物:联苯-双(咪唑) 。用二氧化铅氧化所得咪唑定量产生稳定的共轭苯氧基-硝酰基单基和双基,它们作为电活性顺磁材料而受到关注。单自由基 2,6-二叔丁基-4-[1-氧化-4-(联苯-4-基)-5,5-二甲基-1 H -咪唑-2(5 H ) 的晶体结构研究了[亚基]环己-2,5-二烯酮及其磁化率、所得杂化自由基在溶液中的EPR谱以及4 H-咪唑的循环伏安特性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号