Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Key Role of Lanthanum Oxychloride: Promotional Effects of Lanthanum in NiLaOy/NaCl for Hydrogen Production from Ethyl Acetate and Water
Small ( IF 13.0 ) Pub Date : 2018-07-20 , DOI: 10.1002/smll.201800927 Zhiwei Xue 1 , Yuesong Shen 1 , Peiwen Li 2 , Yu Zhang 1 , Junjie Li 1 , Bin Qin 1 , Jin Zhang 3 , Yanwei Zeng 1 , Shemin Zhu 1
Small ( IF 13.0 ) Pub Date : 2018-07-20 , DOI: 10.1002/smll.201800927 Zhiwei Xue 1 , Yuesong Shen 1 , Peiwen Li 2 , Yu Zhang 1 , Junjie Li 1 , Bin Qin 1 , Jin Zhang 3 , Yanwei Zeng 1 , Shemin Zhu 1
Affiliation
|
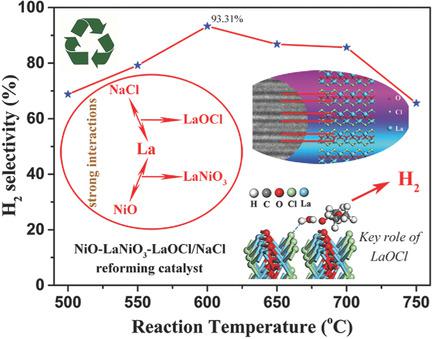
The hydrogen economy is accelerating technological evolutions toward highly efficient hydrogen production. In this work, the catalytic performance of NiO/NaCl for hydrogen production via autothermal reforming of ethyl acetate and water is further improved through lanthanum modification, and the resulted 3%‐NiLaOy/NaCl catalyst achieves as high as 93% H2 selectivity and long‐term stability at 600 °C. The promoting effect is caused by the strong interactions between lanthanum and NiO/NaCl, by which LaNiO3 and a novel LaOCl phase are formed. The key role of LaOCl in promoting low‐temperature hydrogen production is highlighted, while effects of LaNiO3 are well known. The LaOCl (010) facet possesses high adsorption capacity toward co‐chemisorbing ethyl acetate and water. LaOCl strongly interacts with ethyl acetate and H2O in the form of hydrogen bonding and coordination effect. The interactions induce tensions inside ethyl acetate and H2O, activate the molecules, and hence decrease the energy barrier for reaction. In situ Fourier transform infrared spectroscopy (FTIR) reveals that LaOCl along with NaCl enhances the adsorption ability of NiO/NaCl. Moreover, LaOCl improves the dispersion of Ni species in NiO–LaNiO3–LaOCl nanosheets, which possess abundant active sites. The effects together promote hydrogen evolution. Furthermore, the NiLaOy/NaCl catalyst can be easily reborn after deactivation due to the water solubility of NaCl.
中文翻译:

氯氧化镧的关键作用:镧在NiLaOy / NaCl中对乙酸乙酯和水制氢的促进作用
氢经济正在加速向高效氢生产的技术发展。在这项工作中,通过镧改性进一步改善了NiO / NaCl通过乙酸乙酯和水的自热重整制氢制氢的催化性能,得到的3%-NiLaO y / NaCl催化剂可实现高达93%的H 2选择性和在600°C下具有长期稳定性。促进作用是由镧与NiO / NaCl之间的强相互作用引起的,由此形成LaNiO 3和新型LaOCl相。强调了LaOCl在促进低温制氢中的关键作用,而LaNiO 3的作用是众所周知的。LaOCl(010)面对乙酸乙酯和水的共化学吸附具有很高的吸附能力。LaOCl以氢键和配位作用的形式与乙酸乙酯和H 2 O强烈相互作用。相互作用引起乙酸乙酯和H 2 O内部的张力,激活分子,因此降低了反应的能垒。原位傅立叶变换红外光谱(FTIR)显示LaOCl与NaCl一起增强了NiO / NaCl的吸附能力。此外,LaOCl改善了NiO在具有丰富活性位点的NiO–LaNiO 3 –LaOCl纳米片中的分散。这些作用共同促进了氢的释放。此外,NiLaO y由于NaCl的水溶性,/ NaCl催化剂在失活后很容易重生。
更新日期:2018-07-20
中文翻译:

氯氧化镧的关键作用:镧在NiLaOy / NaCl中对乙酸乙酯和水制氢的促进作用
氢经济正在加速向高效氢生产的技术发展。在这项工作中,通过镧改性进一步改善了NiO / NaCl通过乙酸乙酯和水的自热重整制氢制氢的催化性能,得到的3%-NiLaO y / NaCl催化剂可实现高达93%的H 2选择性和在600°C下具有长期稳定性。促进作用是由镧与NiO / NaCl之间的强相互作用引起的,由此形成LaNiO 3和新型LaOCl相。强调了LaOCl在促进低温制氢中的关键作用,而LaNiO 3的作用是众所周知的。LaOCl(010)面对乙酸乙酯和水的共化学吸附具有很高的吸附能力。LaOCl以氢键和配位作用的形式与乙酸乙酯和H 2 O强烈相互作用。相互作用引起乙酸乙酯和H 2 O内部的张力,激活分子,因此降低了反应的能垒。原位傅立叶变换红外光谱(FTIR)显示LaOCl与NaCl一起增强了NiO / NaCl的吸附能力。此外,LaOCl改善了NiO在具有丰富活性位点的NiO–LaNiO 3 –LaOCl纳米片中的分散。这些作用共同促进了氢的释放。此外,NiLaO y由于NaCl的水溶性,/ NaCl催化剂在失活后很容易重生。











































 京公网安备 11010802027423号
京公网安备 11010802027423号