当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Identification and biological evaluation of glycol diaryl ethers as novel anti‐cancer agents through structure‐based optimization of crizotinib
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2018-08-26 , DOI: 10.1111/cbdd.13368 Shasha Liu 1 , Xiaoxia Liu 1 , Xun Zhang 1 , Meiyun Shi 1 , Hecheng Wang 1 , Yajun Liu 1
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2018-08-26 , DOI: 10.1111/cbdd.13368 Shasha Liu 1 , Xiaoxia Liu 1 , Xun Zhang 1 , Meiyun Shi 1 , Hecheng Wang 1 , Yajun Liu 1
Affiliation
|
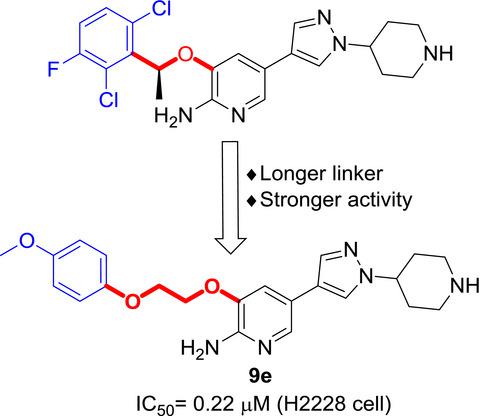
Crizotinib, a drug for anaplastic lymphoma kinase (ALK) positive and c‐ros oncogene 1 receptor tyrosine kinase (ROS1) positive non‐small cell lung cancer (NSCLC), was structurally optimized via a strategy of structure‐based fragment replacing. Computational study showed it was beneficial for interaction of crizotinib and ALK to increase the distance between pyridyl ring and phenyl ring in crizotinib, and thus, a series of novel glycol diaryl ethers were synthesized. The in vitro anti‐tumor activity of synthesized compounds was studied in NSCLC cell line H2228 and neurobalstoma cell line SH‐SY5Y. Among the synthesized compounds, 9e exhibits stronger anti‐cancer activity than crizotinib toward H2228 cell line with an IC50 value of 0.22 μM. Molecular docking indicated that a longer chain between pyridyl ring and phenyl ring enabled molecule to have new interaction with a neighboring small hydrophobic pocket.
中文翻译:

通过基于克唑替尼的结构优化,鉴定乙二醇二芳基醚作为新型抗癌药并对其进行生物学评估
Crizotinib是一种用于间变性淋巴瘤激酶(ALK)阳性和c-ros癌基因1受体酪氨酸激酶(ROS1)阳性非小细胞肺癌(NSCLC)的药物,通过基于结构的片段替换策略在结构上进行了优化。计算研究表明,增加克唑替尼中吡啶环与苯环的距离有利于克唑替尼与ALK的相互作用,从而合成了一系列新颖的乙二醇二芳基醚。在NSCLC细胞系H2228和神经造口瘤细胞系SH-SY5Y中研究了合成化合物的体外抗肿瘤活性。在合成的化合物中,9e对克洛替尼具有IC 50的H2228细胞系具有更强的抗癌活性值为0.22μM。分子对接表明,吡啶基环和苯环之间的较长链使分子能够与相邻的小疏水口袋形成新的相互作用。
更新日期:2018-08-26
中文翻译:

通过基于克唑替尼的结构优化,鉴定乙二醇二芳基醚作为新型抗癌药并对其进行生物学评估
Crizotinib是一种用于间变性淋巴瘤激酶(ALK)阳性和c-ros癌基因1受体酪氨酸激酶(ROS1)阳性非小细胞肺癌(NSCLC)的药物,通过基于结构的片段替换策略在结构上进行了优化。计算研究表明,增加克唑替尼中吡啶环与苯环的距离有利于克唑替尼与ALK的相互作用,从而合成了一系列新颖的乙二醇二芳基醚。在NSCLC细胞系H2228和神经造口瘤细胞系SH-SY5Y中研究了合成化合物的体外抗肿瘤活性。在合成的化合物中,9e对克洛替尼具有IC 50的H2228细胞系具有更强的抗癌活性值为0.22μM。分子对接表明,吡啶基环和苯环之间的较长链使分子能够与相邻的小疏水口袋形成新的相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号