当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fluorescent Sulphur‐ and Nitrogen‐Containing Porous Polymers with Tuneable Donor–Acceptor Domains for Light‐Driven Hydrogen Evolution
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-07-25 , DOI: 10.1002/chem.201802902 Dana Schwarz 1, 2 , Amitava Acharja 3 , Arun Ichangi 1, 4 , Pengbo Lyu 5 , Maksym V. Opanasenko 5 , Fabian R. Goßler 2 , Tobias A. F. König 2 , Jiří Čejka 5 , Petr Nachtigall 5 , Arne Thomas 3 , Michael J. Bojdys 4, 6
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-07-25 , DOI: 10.1002/chem.201802902 Dana Schwarz 1, 2 , Amitava Acharja 3 , Arun Ichangi 1, 4 , Pengbo Lyu 5 , Maksym V. Opanasenko 5 , Fabian R. Goßler 2 , Tobias A. F. König 2 , Jiří Čejka 5 , Petr Nachtigall 5 , Arne Thomas 3 , Michael J. Bojdys 4, 6
Affiliation
|
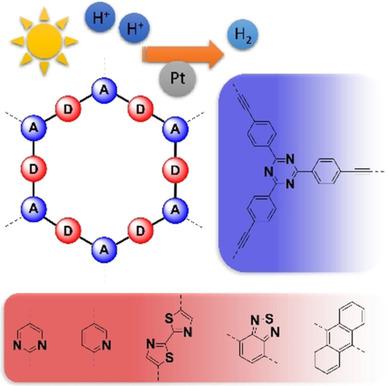
Light‐driven water splitting is a potential source of abundant, clean energy, yet efficient charge‐separation and size and position of the bandgap in heterogeneous photocatalysts are challenging to predict and design. Synthetic attempts to tune the bandgap of polymer photocatalysts classically rely on variations of the sizes of their π‐conjugated domains. However, only donor–acceptor dyads hold the key to prevent undesired electron‐hole recombination within the catalyst via efficient charge separation. Building on our previous success in incorporating electron‐donating, sulphur‐containing linkers and electron‐withdrawing, triazine (C3N3) units into porous polymers, we report the synthesis of six visible‐light‐active, triazine‐based polymers with a high heteroatom‐content of S and N that photocatalytically generate H2 from water: up to 915 μmol h−1 g−1 with Pt co‐catalyst, and—as one of the highest to‐date reported values −200 μmol h−1 g−1 without. The highly modular Sonogashira–Hagihara cross‐coupling reaction we employ, enables a systematic study of mixed (S, N, C) and (N, C)‐only polymer systems. Our results highlight that photocatalytic water‐splitting does not only require an ideal optical bandgap of ≈2.2 eV, but that the choice of donor–acceptor motifs profoundly impacts charge‐transfer and catalytic activity.
中文翻译:

具有可调供体-受体域的荧光型含硫和氮的多孔聚合物,用于光驱氢的演化
光驱动的水分解是潜在的丰富,清洁能源的来源,但是要有效地进行电荷分离以及带隙在多相光催化剂中的大小和位置,要进行预测和设计都充满了挑战。调节聚合物光催化剂带隙的合成尝试通常依赖于其π共轭结构域大小的变化。然而,只有施主-受主二元才是通过有效的电荷分离来防止催化剂内不希望的电子-空穴复合的关键。在我们先前成功地将供电子,含硫连接子和吸电子三嗪(C 3 N 3)单元成多孔聚合物,我们报告了六种具有高杂原子含量的S和N的可见光活性三嗪基聚合物的合成,这些聚合物可光催化从水中产生H 2:高达915μmolh -1 g -1含Pt助催化剂,并且-迄今报道的最高值之一-200μmolh -1 g -1无。我们采用了高度模块化的Sonogashira–Ha原交叉偶联反应,可以对仅含(S,N,C)和(N,C)混合聚合物系统进行系统研究。我们的结果表明,光催化水分解不仅需要约2.2 eV的理想光学带隙,而且供体-受体基序的选择会深刻影响电荷转移和催化活性。
更新日期:2018-07-25
中文翻译:

具有可调供体-受体域的荧光型含硫和氮的多孔聚合物,用于光驱氢的演化
光驱动的水分解是潜在的丰富,清洁能源的来源,但是要有效地进行电荷分离以及带隙在多相光催化剂中的大小和位置,要进行预测和设计都充满了挑战。调节聚合物光催化剂带隙的合成尝试通常依赖于其π共轭结构域大小的变化。然而,只有施主-受主二元才是通过有效的电荷分离来防止催化剂内不希望的电子-空穴复合的关键。在我们先前成功地将供电子,含硫连接子和吸电子三嗪(C 3 N 3)单元成多孔聚合物,我们报告了六种具有高杂原子含量的S和N的可见光活性三嗪基聚合物的合成,这些聚合物可光催化从水中产生H 2:高达915μmolh -1 g -1含Pt助催化剂,并且-迄今报道的最高值之一-200μmolh -1 g -1无。我们采用了高度模块化的Sonogashira–Ha原交叉偶联反应,可以对仅含(S,N,C)和(N,C)混合聚合物系统进行系统研究。我们的结果表明,光催化水分解不仅需要约2.2 eV的理想光学带隙,而且供体-受体基序的选择会深刻影响电荷转移和催化活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号