当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrophilic carbocyclization reactions of 2-(2-alkynylphenyl)amino-1,4-naphthoquinones†
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-07-19 00:00:00 , DOI: 10.1039/c8ob01170b Chang-You Sie,Che-Ping Chuang
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-07-19 00:00:00 , DOI: 10.1039/c8ob01170b Chang-You Sie,Che-Ping Chuang
|
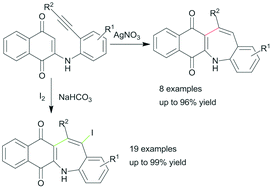
An efficient Ag(I)-catalyzed method for the synthesis of 5H-benzo[b]naphtho[2,3-f]azepine-6,11-diones from 2-(2-ethynylphenyl)amino substituted 1,4-naphthoquinones has been developed. In this transformation new C–C bond formation occurred regioselectively via a 7-endo-dig cyclization. The related 13-iodine substituted benzazepine derivatives could also be produced exclusively by the I2-induced carbocyclization reaction of 2-(2-alkynylphenyl)amino substituted 1,4-naphthoquinones. This process has advantages such as mild reaction conditions and tolerance of a broad range of functional groups.
中文翻译:

2-(2-炔基苯基)氨基-1,4-萘醌的亲电碳环化反应†
由2-(2-乙炔基苯基)氨基取代的1,4-萘醌合成5 H-苯并[ b ]萘并[2,3 - f ]氮杂-6,11-二酮的有效Ag(I)催化方法已经被开发出来。在这种转化中,新的CC键通过7-内切环化在区域上选择性地发生。相关的13-碘取代的苯并ze庚因衍生物也可以仅通过I 2诱导的2-(2-炔基苯基)氨基取代的1,4-萘醌的碳环化反应来生产。该方法具有诸如温和的反应条件和对各种官能团的耐受性的优点。
更新日期:2018-07-19
中文翻译:

2-(2-炔基苯基)氨基-1,4-萘醌的亲电碳环化反应†
由2-(2-乙炔基苯基)氨基取代的1,4-萘醌合成5 H-苯并[ b ]萘并[2,3 - f ]氮杂-6,11-二酮的有效Ag(I)催化方法已经被开发出来。在这种转化中,新的CC键通过7-内切环化在区域上选择性地发生。相关的13-碘取代的苯并ze庚因衍生物也可以仅通过I 2诱导的2-(2-炔基苯基)氨基取代的1,4-萘醌的碳环化反应来生产。该方法具有诸如温和的反应条件和对各种官能团的耐受性的优点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号