Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel indole based hybrid oxadiazole scaffolds with N-(substituted-phenyl)butanamides: synthesis, lineweaver–burk plot evaluation and binding analysis of potent urease inhibitors†
RSC Advances ( IF 3.9 ) Pub Date : 2018-07-19 00:00:00 , DOI: 10.1039/c8ra04987d Majid Nazir 1 , Muhammad Athar Abbasi 1, 2 , Aziz-Ur-Rehman 1 , Sabahat Zahra Siddiqui 1 , Hussain Raza 2 , Mubashir Hassan 2 , Syed Adnan Ali Shah 3 , Muhammad Shahid 4 , Sung-Yum Seo 2
RSC Advances ( IF 3.9 ) Pub Date : 2018-07-19 00:00:00 , DOI: 10.1039/c8ra04987d Majid Nazir 1 , Muhammad Athar Abbasi 1, 2 , Aziz-Ur-Rehman 1 , Sabahat Zahra Siddiqui 1 , Hussain Raza 2 , Mubashir Hassan 2 , Syed Adnan Ali Shah 3 , Muhammad Shahid 4 , Sung-Yum Seo 2
Affiliation
|
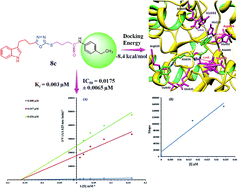
In the study presented herein, 4-(1H-indol-3-yl)butanoic acid (1) was sequentially transformed in the first phase into ethyl 4-(1H-indol-3-yl)butanoate (2), 4-(1H-indol-3-yl)butanohydrazide (3) and 5-[3-(1H-indol-3-yl)propyl]-1,3,4-oxadiazole-2-thiol (4) as a nucleophile. In the second phase, various electrophiles were synthesized by reacting substituted-anilines, 5a–j, with 4-chlorobutanoyl chloride (6) to afford 4-chloro-N-(substituted-phenyl)butanamides (7a–j). In the final phase, nucleophilic substitution reaction of 4 was carried out with different electrophiles, 7a–j, to achieve novel indole based oxadiazole scaffolds with N-(substituted-phenyl)butamides (8a–j). The structural confirmation of all the as-synthesized compounds was performed by spectral and elemental analysis. These molecules were screened for their in vitro inhibitory potential against urease enzyme and were found to be potent inhibitors. The results of enzyme inhibitory kinetics showed that compound 8c inhibited the enzyme competitively with a Ki value 0.003 μM. The results of the in silico study of these scaffolds were in full agreement with the experimental data and the ligands showed good binding energy values. The hemolytic study revealed their mild cytotoxicity towards cell membranes and hence, these molecules can be regarded as valuable therapeutic agents in drug designing programs.
中文翻译:

具有 N-(取代苯基)丁酰胺的新型吲哚基杂化恶二唑支架:有效脲酶抑制剂的合成、lineweaver-burk 图评估和结合分析†
在本文介绍的研究中,4-(1 H -indol-3-yl)butanoic acid ( 1 ) 在第一阶段被顺序转化为 4-( 1H -indol-3-yl)butanoate ( 2 ), 4 -(1 H-吲哚-3-基)丁酰肼 ( 3 ) 和 5-[3-(1 H-吲哚-3-基)丙基]-1,3,4-恶二唑-2-硫醇 ( 4 )亲核试剂。在第二阶段,通过取代苯胺5a-j与 4-氯丁酰氯 ( 6 ) 反应合成各种亲电试剂,得到 4-氯代-N- (取代苯基)丁酰胺 ( 7a-j )。在最后阶段,亲核取代反应图4是用不同的亲电试剂7a-j进行的,以实现具有N-(取代苯基)丁酰胺的新型吲哚基恶二唑支架(8a-j)。通过光谱和元素分析对所有合成后的化合物进行结构确认。筛选了这些分子对脲酶的体外抑制潜力,发现它们是有效的抑制剂。酶抑制动力学结果表明,化合物8c竞争性抑制酶,K i值为0.003 μM。计算机模拟结果这些支架的研究与实验数据完全一致,并且配体显示出良好的结合能值。溶血研究揭示了它们对细胞膜的轻微细胞毒性,因此,这些分子可被视为药物设计程序中有价值的治疗剂。
更新日期:2018-07-19
中文翻译:

具有 N-(取代苯基)丁酰胺的新型吲哚基杂化恶二唑支架:有效脲酶抑制剂的合成、lineweaver-burk 图评估和结合分析†
在本文介绍的研究中,4-(1 H -indol-3-yl)butanoic acid ( 1 ) 在第一阶段被顺序转化为 4-( 1H -indol-3-yl)butanoate ( 2 ), 4 -(1 H-吲哚-3-基)丁酰肼 ( 3 ) 和 5-[3-(1 H-吲哚-3-基)丙基]-1,3,4-恶二唑-2-硫醇 ( 4 )亲核试剂。在第二阶段,通过取代苯胺5a-j与 4-氯丁酰氯 ( 6 ) 反应合成各种亲电试剂,得到 4-氯代-N- (取代苯基)丁酰胺 ( 7a-j )。在最后阶段,亲核取代反应图4是用不同的亲电试剂7a-j进行的,以实现具有N-(取代苯基)丁酰胺的新型吲哚基恶二唑支架(8a-j)。通过光谱和元素分析对所有合成后的化合物进行结构确认。筛选了这些分子对脲酶的体外抑制潜力,发现它们是有效的抑制剂。酶抑制动力学结果表明,化合物8c竞争性抑制酶,K i值为0.003 μM。计算机模拟结果这些支架的研究与实验数据完全一致,并且配体显示出良好的结合能值。溶血研究揭示了它们对细胞膜的轻微细胞毒性,因此,这些分子可被视为药物设计程序中有价值的治疗剂。










































 京公网安备 11010802027423号
京公网安备 11010802027423号