当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermodynamic Scale of β-Amino Acid Residue Propensities for an α-Helix-like Conformation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-07-19 , DOI: 10.1021/jacs.8b05162 Brian F Fisher 1 , Seong Ho Hong 1 , Samuel H Gellman 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-07-19 , DOI: 10.1021/jacs.8b05162 Brian F Fisher 1 , Seong Ho Hong 1 , Samuel H Gellman 1
Affiliation
|
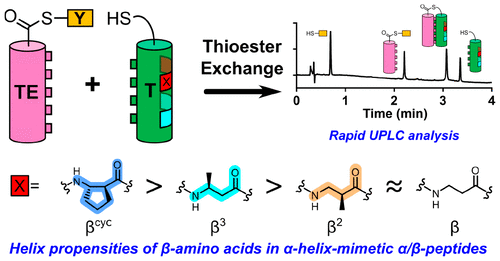
A thiol-thioester exchange system has been used to measure the propensities of diverse β-amino acid residues to participate in an α-helix-like conformation. These measurements depend on formation of a parallel coiled-coil tertiary structure when two peptide segments become linked by thioester formation. One peptide segment contains a "guest" site that accommodates diverse β residues and is distal to the coiled-coil interface. We find that helix propensity is influenced by side chain placement within the β residue [β3 (side chain adjacent to nitrogen) slightly favored relative to β2 (side chain adjacent to carbonyl)]. The previously recognized helix stabilization resulting from five-membered ring incorporation is quantified. These results are significant because so few quantitative thermodynamic measurements have been reported for α/β-peptide folding.
中文翻译:

α-螺旋样构象的 β-氨基酸残基倾向的热力学尺度
硫醇-硫酯交换系统已用于测量不同β-氨基酸残基参与α-螺旋样构象的倾向。这些测量取决于当两个肽片段通过硫酯形成连接时平行卷曲螺旋三级结构的形成。一个肽片段含有一个“客体”位点,可容纳不同的β残基,并且位于卷曲螺旋界面的远端。我们发现螺旋倾向受到β残基内侧链位置的影响[β3(与氮相邻的侧链)相对于β2(与羰基相邻的侧链)稍微有利]。先前认识到的由五元环结合产生的螺旋稳定性已被量化。这些结果意义重大,因为关于 α/β-肽折叠的定量热力学测量报道很少。
更新日期:2018-07-19
中文翻译:

α-螺旋样构象的 β-氨基酸残基倾向的热力学尺度
硫醇-硫酯交换系统已用于测量不同β-氨基酸残基参与α-螺旋样构象的倾向。这些测量取决于当两个肽片段通过硫酯形成连接时平行卷曲螺旋三级结构的形成。一个肽片段含有一个“客体”位点,可容纳不同的β残基,并且位于卷曲螺旋界面的远端。我们发现螺旋倾向受到β残基内侧链位置的影响[β3(与氮相邻的侧链)相对于β2(与羰基相邻的侧链)稍微有利]。先前认识到的由五元环结合产生的螺旋稳定性已被量化。这些结果意义重大,因为关于 α/β-肽折叠的定量热力学测量报道很少。











































 京公网安备 11010802027423号
京公网安备 11010802027423号