当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Elucidation of Chemical Species and Reactivity at Methylammonium Lead Iodide and Cesium Tin Bromide Perovskite Surfaces via Orthogonal Reaction Chemistry
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-08-01 , DOI: 10.1021/acs.jpcc.8b05352 Weiran Gao 1 , Kenneth Zielinski 1 , Benjamin N. Drury 1 , Alexander D. Carl 1 , Ronald L. Grimm 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-08-01 , DOI: 10.1021/acs.jpcc.8b05352 Weiran Gao 1 , Kenneth Zielinski 1 , Benjamin N. Drury 1 , Alexander D. Carl 1 , Ronald L. Grimm 1
Affiliation
|
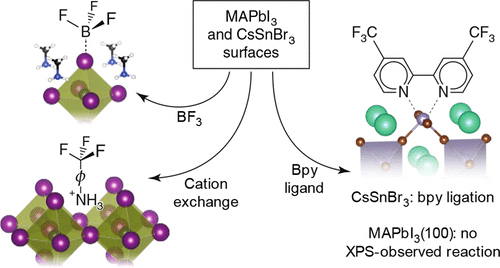
We quantified the chemical species present at and reactivity of the (100) face of tetrahedral single-crystal methylammonium lead iodide, MAPbI3(100), and polycrystalline cesium tin bromide, CsSnBr3. For these ABX3 perovskites, experiments utilized the orthogonal reactivity of the A+-site cation, the B2+-site cation, and the X–-site halide anion. Ambient pressure exposure to BF3 solutions probed the reactivity of interfacial halides. Reactions with p-trifluoromethylanilinium chloride probed the exchange reactivity of the A+-site cation. A complex-forming ligand, 4,4′-bis(trifluoromethyl)-2,2′-bipyridine, probed for interfacial B2+-site cations. Fluorine features in X-ray photoelectron spectroscopy (XPS) quantified reaction outcomes for each solution-phase species. XPS revealed adsorption of BF3, indicating surface-available halide anions on both MAPbI3(100) and on CsSnBr3. Temperature-programmed desorption quantified a ∼200 kJ mol–1 desorption activation energy from MAPbI3(100) and a ∼215 kJ mol–1 desorption energy from CsSnBr3. Adsorption of the fluorinated anilinium cation included no concomitant adsorption of chlorine as revealed by the absence of Cl 2p features within the limits of XPS detection. We interpret the observation of the anilinium species as exchanging for interfacial methylammonium species on MAPbI3(100) surfaces and interfacial cesium on the polycrystalline CsSnBr3 surface. Within detection limits, the bipyridine ligand demonstrated no adsorption to MAPbI3(100), suggestive of a Pb2+ deficient surface, but adsorption to the polycrystalline CsSnBr3 that suggests surface-accessible Sn2+. The combination of results implies that methylammonium cations and iodide anions dominate tetragonal MAPbI3(100) surface that, respectively, enables cation exchange and Lewis adduct formation for surface derivatization. We discuss the present results in the context of interfacial stability, passivation, and reactivity for perovskite-based energy conversion.
中文翻译:

通过正交反应化学阐明甲基铵碘化铅和溴化铯锡钙钛矿表面的化学物种和反应性
我们量化的化学物种出席和四面体单晶甲基铵碘化铅的(100)面的反应性,MAPbI 3(100),和多晶铯溴化锡,CsSnBr 3。对于这些ABX 3钙钛矿,实验中使用的A的正交反应性+上门阳离子中,B 2+上门阳离子,和X - -site卤化物阴离子。环境压力暴露于BF 3溶液可探测界面卤化物的反应性。与对-三氟甲基苯胺氯化物的反应探测了A +的交换反应性位阳离子。形成络合物的配体4,4'-双(三氟甲基)-2,2'-联吡啶检测出界面B 2+位阳离子。X射线光电子能谱(XPS)中的氟特征量化了每种溶液相物质的反应结果。XPS揭示了BF 3的吸附,表明MAPbI 3(100)和CsSnBr 3上均存在表面可用的卤化物阴离子。程序升温脱附量化一个〜200千焦耳摩尔-1从MAPbI脱附活化能3(100)和一个~215千焦耳摩尔-1从CsSnBr解吸能量3。氟苯胺阳离子的吸附不包括氯的伴随吸附,这是由于在XPS检测限度内不存在Cl 2p特征而显示的。我们将对苯胺物种的观察解释为交换了MAPbI 3(100)表面上的界面甲基铵物种和多晶CsSnBr 3表面上的界面铯。在检测限内,联吡啶配体未显示对MAPbI 3的吸附(100),表明存在Pb 2+缺陷表面,但对多晶CsSnBr 3的吸附表明表面可接触Sn 2+。结果的组合表明,甲基铵阳离子和碘化物阴离子支配四方MAPbI 3(100)表面,这分别使阳离子交换和形成路易斯的加合物形成表面即可。我们在界面稳定性,钝化和基于钙钛矿的能量转化的反应性的背景下讨论本研究结果。
更新日期:2018-08-02
中文翻译:

通过正交反应化学阐明甲基铵碘化铅和溴化铯锡钙钛矿表面的化学物种和反应性
我们量化的化学物种出席和四面体单晶甲基铵碘化铅的(100)面的反应性,MAPbI 3(100),和多晶铯溴化锡,CsSnBr 3。对于这些ABX 3钙钛矿,实验中使用的A的正交反应性+上门阳离子中,B 2+上门阳离子,和X - -site卤化物阴离子。环境压力暴露于BF 3溶液可探测界面卤化物的反应性。与对-三氟甲基苯胺氯化物的反应探测了A +的交换反应性位阳离子。形成络合物的配体4,4'-双(三氟甲基)-2,2'-联吡啶检测出界面B 2+位阳离子。X射线光电子能谱(XPS)中的氟特征量化了每种溶液相物质的反应结果。XPS揭示了BF 3的吸附,表明MAPbI 3(100)和CsSnBr 3上均存在表面可用的卤化物阴离子。程序升温脱附量化一个〜200千焦耳摩尔-1从MAPbI脱附活化能3(100)和一个~215千焦耳摩尔-1从CsSnBr解吸能量3。氟苯胺阳离子的吸附不包括氯的伴随吸附,这是由于在XPS检测限度内不存在Cl 2p特征而显示的。我们将对苯胺物种的观察解释为交换了MAPbI 3(100)表面上的界面甲基铵物种和多晶CsSnBr 3表面上的界面铯。在检测限内,联吡啶配体未显示对MAPbI 3的吸附(100),表明存在Pb 2+缺陷表面,但对多晶CsSnBr 3的吸附表明表面可接触Sn 2+。结果的组合表明,甲基铵阳离子和碘化物阴离子支配四方MAPbI 3(100)表面,这分别使阳离子交换和形成路易斯的加合物形成表面即可。我们在界面稳定性,钝化和基于钙钛矿的能量转化的反应性的背景下讨论本研究结果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号