Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Three-dimensional mesoporous calcium carbonate–silica frameworks thermally activated from porous fossil bryophyte: adsorption studies for heavy metal uptake
RSC Advances ( IF 3.9 ) Pub Date : 2018-07-18 00:00:00 , DOI: 10.1039/c8ra04825h Wenlei Wang 1 , Ren He 1 , Tianli Yang 1 , Yunchu Hu 1 , Ning Zhang 1 , Can Yang 1
RSC Advances ( IF 3.9 ) Pub Date : 2018-07-18 00:00:00 , DOI: 10.1039/c8ra04825h Wenlei Wang 1 , Ren He 1 , Tianli Yang 1 , Yunchu Hu 1 , Ning Zhang 1 , Can Yang 1
Affiliation
|
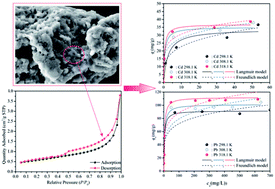
In this paper, three-dimensional mesoporous calcium carbonate–silica frameworks have been created from the straw tufa (ST) originating from porous fossil bryophyte by a thermal activation technique. A batch of adsorption kinetic and thermodynamic experiments were used to investigate the adsorption capacity of Cd(II) onto the samples. The ST after thermal activation has shown a significant ability for the uptake of heavy metals. It exhibited maximum adsorption capacities of 12.76 mg g−1, 14.09 mg g−1, 17.00 mg g−1, and 33.81 mg g−1 for Cd(II) at the activation temperature of 300, 450, 600 and 750 °C, respectively. Through competitive adsorption for Cd(II)and Pb(II), the ST thermally activated at 750 °C exhibited maximum equilibrium adsorption capacities of 24.65 mg g−1, 25.91 mg g−1, and 30.94 mg g−1 for Cd(II) uptake at 298.1 K, 308.1 K and 318.1 K, respectively, whereas it exhibited values of 91.59 mg g−1, 101.32 mg g−1, and 112.19 mg g−1 for Pb(II) removal. The adsorption capacities of Cd(II) and Pb(II) both decrease with the addition of the other heavy metal cations, indicating that the adsorption is hindered by the competitive adsorption and the adsorption active sites on the mineral surface are readily exchangeable. The adsorption of Cd(II) and Pb(II) followed the pseudo-second order kinetics model well. In addition, the Langmuir model could accurately describe the adsorption isotherms. Based on the results of characterization with TEM, XRD and XPS, the adsorption mechanisms could be primarily explained as formation of Cd(OH)2 and CdCO3 as well as Cd(HCO3)2 precipitation on the surface of ST. These characteristics of ion-exchange and the adsorptive property for ST modified allow it to be widely used in artificial wetland landfill and environmental protection.
中文翻译:

多孔苔藓植物化石热活化的三维介孔碳酸钙-二氧化硅骨架:重金属吸收的吸附研究
在本文中,通过热活化技术从源自多孔化石苔藓植物的秸秆凝灰岩(ST)创建了三维介孔碳酸钙-二氧化硅骨架。一批吸附动力学和热力学实验被用来研究Cd( II )对样品的吸附能力。热活化后的 ST 显示出对重金属吸收的显着能力。在 300、450、600和 750 °C 的活化温度下,它对Cd( II ) 的最大吸附容量分别为 12.76 mg g -1、14.09 mg g -1、17.00 mg g -1和 33.81 mg g -1 ,分别。通过竞争吸附 Cd( II) 和 Pb( II ),在 750 °C 热活化的 ST 表现出 24.65 mg g -1、25.91 mg g -1和 30.94 mg g -1的最大平衡吸附容量,在 298.1 K 时吸收 Cd( II ),308.1 K 和 318.1 K 分别为 91.59 mg g -1、101.32 mg g -1和 112.19 mg g -1去除Pb( II )。Cd( II )和Pb( II )的吸附能力) 均随着其他重金属阳离子的加入而降低,表明吸附受到竞争吸附的阻碍,并且矿物表面的吸附活性位点易于交换。Cd( II )和Pb( II )的吸附符合准二级动力学模型。此外,Langmuir 模型可以准确地描述吸附等温线。基于 TEM、XRD 和 XPS 的表征结果,吸附机理可以主要解释为 Cd(OH) 2和 CdCO 3以及 Cd(HCO 3 ) 2的形成。ST表面的沉淀。ST改性的这些离子交换特性和吸附性能使其广泛应用于人工湿地填埋和环境保护。
更新日期:2018-07-18
中文翻译:

多孔苔藓植物化石热活化的三维介孔碳酸钙-二氧化硅骨架:重金属吸收的吸附研究
在本文中,通过热活化技术从源自多孔化石苔藓植物的秸秆凝灰岩(ST)创建了三维介孔碳酸钙-二氧化硅骨架。一批吸附动力学和热力学实验被用来研究Cd( II )对样品的吸附能力。热活化后的 ST 显示出对重金属吸收的显着能力。在 300、450、600和 750 °C 的活化温度下,它对Cd( II ) 的最大吸附容量分别为 12.76 mg g -1、14.09 mg g -1、17.00 mg g -1和 33.81 mg g -1 ,分别。通过竞争吸附 Cd( II) 和 Pb( II ),在 750 °C 热活化的 ST 表现出 24.65 mg g -1、25.91 mg g -1和 30.94 mg g -1的最大平衡吸附容量,在 298.1 K 时吸收 Cd( II ),308.1 K 和 318.1 K 分别为 91.59 mg g -1、101.32 mg g -1和 112.19 mg g -1去除Pb( II )。Cd( II )和Pb( II )的吸附能力) 均随着其他重金属阳离子的加入而降低,表明吸附受到竞争吸附的阻碍,并且矿物表面的吸附活性位点易于交换。Cd( II )和Pb( II )的吸附符合准二级动力学模型。此外,Langmuir 模型可以准确地描述吸附等温线。基于 TEM、XRD 和 XPS 的表征结果,吸附机理可以主要解释为 Cd(OH) 2和 CdCO 3以及 Cd(HCO 3 ) 2的形成。ST表面的沉淀。ST改性的这些离子交换特性和吸附性能使其广泛应用于人工湿地填埋和环境保护。











































 京公网安备 11010802027423号
京公网安备 11010802027423号