当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enzymatic Cross‐Benzoin‐Type Condensation of Aliphatic Aldehydes: Enantioselective Synthesis of 1‐Alkyl‐1‐hydroxypropan‐2‐ones and 1‐Alkyl‐1‐hydroxybutan‐2‐ones
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-08-09 , DOI: 10.1002/adsc.201800357 Graziano Di Carmine 1 , Olga Bortolini 1 , Alessandro Massi 1 , Michael Müller 2 , Giovanni Bernacchia 3 , Giancarlo Fantin 1 , Daniele Ragno 1 , Pier Paolo Giovannini 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-08-09 , DOI: 10.1002/adsc.201800357 Graziano Di Carmine 1 , Olga Bortolini 1 , Alessandro Massi 1 , Michael Müller 2 , Giovanni Bernacchia 3 , Giancarlo Fantin 1 , Daniele Ragno 1 , Pier Paolo Giovannini 1
Affiliation
|
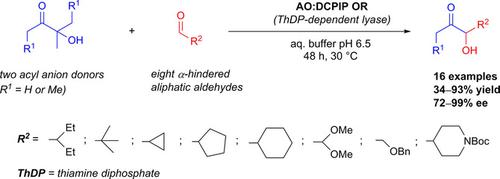
Benzoin‐type reactions have been intensively exploited as a synthetic strategy for the preparation of α‐hydroxy ketones. Thiamine diphosphate (ThDP) dependent enzymes are excellent catalysts for asymmetric versions of such reaction types. In particular, in cross‐benzoin condensations of aromatic reactants and mixed aromatic/aliphatic reactions, use of these enzymes has resulted in high levels of chemo‐, regio‐ and stereoselectivity. The present work, which confirms this trend for aliphatic reactants, outlines results obtained in the formal cross‐benzoin‐type condensation of the ‘umpoled’ acetaldehyde and propionaldehyde with various aliphatic aldehydes catalyzed by the ThDP‐dependent enzyme acetoin:dichlorophenolindophenol oxidoreductase (Ao:DCPIP OR). In these reactions, 3‐hydroxy‐3‐methylbutan‐2‐one (methylacetoin) was used as the activated acetaldehyde donor, while 4‐hydroxy‐4‐methylhexan‐3‐one was employed for the first time as the precursor of activated propionaldehyde. With the exception of 3‐hydroxypentan‐2‐one and 3‐hydroxyhexan‐2‐one, which were obtained in almost racemic form by the condensation of methylacetoin with propanal and butanal, respectively, all other products achieved from reactions performed using the same donor with more hindered aldehyde acceptors were obtained with high conversions (89–99%) and in good to high enantiomeric excess (72–99% ee). In a similar way, high conversions (75–99%) and good ee (76–99%) were observed in reactions performed with the same set of aldehyde acceptors, but using 4‐hydroxy‐4‐methylhexan‐3‐one as propionyl anion donor. This is the first time that most of the products described herein have been prepared via benzoin‐type condensation.
中文翻译:

脂族醛类的酶促跨联苯甲醛缩合反应:1-烷基-1-羟基丙烷-2-酮和1-烷基-1-羟基丁酮-2-酮的对映选择性合成
苯甲素类反应已被广泛用作制备α的合成策略-羟基酮。硫胺二磷酸(ThDP)依赖性酶是此类反应类型的不对称形式的出色催化剂。特别是,在芳族反应物的交叉安息香缩合和芳族/脂肪族混合反应中,这些酶的使用导致了高水平的化学,区域和立体选择性。目前的工作证实了脂族反应物的这一趋势,概述了由ThDP依赖性乙酰丙酮:二氯苯酚吲哚酚氧化还原酶(Ao: DCPIP OR)。在这些反应中,使用3-羟基-3-甲基丁-2-酮(甲基乙酰丁)作为活化的乙醛供体,而4-羟基-4-甲基己基-3-酮是首次用作活化丙醛的前体。除了3-羟基戊烷-2-酮和3-羟基己酮-2-酮分别通过甲基乙酰丙酮分别与丙醛和丁醛缩合而得到外消旋形式外,所有其他产物均是通过使用同一供体进行反应而获得的较高的转化率(89–99%)和对映异构体过量至较高的对映体(72–99%)获得了具有更多受阻醛受体的化合物ee)。以类似的方式,在使用相同的一组醛受体但使用4-羟基-4-甲基己基-3-酮作为丙酰基的反应中,观察到了高转化率(75–99%)和良好的ee(76–99%)。阴离子供体。这是本文所述的大多数产品都是通过安息香型缩合反应首次制备的。
更新日期:2018-08-09
中文翻译:

脂族醛类的酶促跨联苯甲醛缩合反应:1-烷基-1-羟基丙烷-2-酮和1-烷基-1-羟基丁酮-2-酮的对映选择性合成
苯甲素类反应已被广泛用作制备α的合成策略-羟基酮。硫胺二磷酸(ThDP)依赖性酶是此类反应类型的不对称形式的出色催化剂。特别是,在芳族反应物的交叉安息香缩合和芳族/脂肪族混合反应中,这些酶的使用导致了高水平的化学,区域和立体选择性。目前的工作证实了脂族反应物的这一趋势,概述了由ThDP依赖性乙酰丙酮:二氯苯酚吲哚酚氧化还原酶(Ao: DCPIP OR)。在这些反应中,使用3-羟基-3-甲基丁-2-酮(甲基乙酰丁)作为活化的乙醛供体,而4-羟基-4-甲基己基-3-酮是首次用作活化丙醛的前体。除了3-羟基戊烷-2-酮和3-羟基己酮-2-酮分别通过甲基乙酰丙酮分别与丙醛和丁醛缩合而得到外消旋形式外,所有其他产物均是通过使用同一供体进行反应而获得的较高的转化率(89–99%)和对映异构体过量至较高的对映体(72–99%)获得了具有更多受阻醛受体的化合物ee)。以类似的方式,在使用相同的一组醛受体但使用4-羟基-4-甲基己基-3-酮作为丙酰基的反应中,观察到了高转化率(75–99%)和良好的ee(76–99%)。阴离子供体。这是本文所述的大多数产品都是通过安息香型缩合反应首次制备的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号