当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Investigating the Low Temperature Formation of CuII‐(N,O) Species on Cu‐CHA Zeolites for the Selective Catalytic Reduction of NOx
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2018-07-26 , DOI: 10.1002/chem.201802769 Chiara Negri 1 , Peter S. Hammershøi 2, 3 , Ton V. W. Janssens 2 , Pablo Beato 4 , Gloria Berlier 1 , Silvia Bordiga 1
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2018-07-26 , DOI: 10.1002/chem.201802769 Chiara Negri 1 , Peter S. Hammershøi 2, 3 , Ton V. W. Janssens 2 , Pablo Beato 4 , Gloria Berlier 1 , Silvia Bordiga 1
Affiliation
|
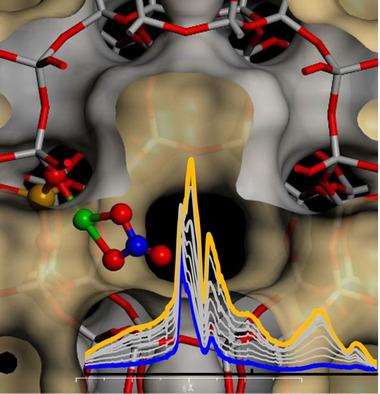
In this work, we show the potentiality of operando FTIR spectroscopy to follow the formation of CuII‐(N,O) species on Cu exchanged chabazite zeolites (Cu‐CHA), active for the selective catalytic reduction of NOx with NH3 (NH3‐SCR). In particular, we investigated the reaction of NO and O2 at low temperature (200 and 50 °C) on a series of Cu‐CHA zeolites with different composition (Si/Al and Cu/Al ratios), to investigate the nature of the formed copper nitrates, which have been proposed to be key intermediates in the oxidation part of the SCR cycle. Our results show that chelating bidentate nitrates are the main structures formed at 200 °C. At lower temperature a mixture of chelating and monodentate nitrates are formed, together with the nitrosonium ion NO+, whose amount was found to be proportional to the zeolite Brønsted site concentration. Nitrates were found to mainly form with CuII ions stabilized by one negative framework charge (Z), Z‐[Cu(OH]I or Z‐[Cu(O2]I, without involvement of Z2‐CuII ones. This evidence, together with the absence of bridging nitrates in samples with high probability for Cu–Cu pairs, indicate that the nitrate ligands are not able to mobilize copper ions, at variance with what recently reported for NH3. Finally, water was found to replace preformed chelating copper nitrates and deplete NO+ (though with different kinetics) at both temperatures, while favouring the presence of monodentate ones.
中文翻译:

研究Cu-CHA沸石上CuII-(N,O)物种的低温形成,以选择性催化还原NOx
在这项工作中,我们显示operando FTIR的潜力光谱分析遵循形成Cu II - (N,O)对Cu物种交换的菱沸石的沸石法(Cu-CHA),活性为选择性催化还原NO的X与NH 3( NH 3 -SCR)。特别是,我们研究了NO和O 2的反应在低温下(200和50°C)在一系列具有不同成分(Si / Al和Cu / Al比)的Cu-CHA沸石上研究形成的硝酸铜的性质,这些硝酸铜被认为是关键的中间体在SCR循环的氧化部分。我们的结果表明,螯合的双齿硝酸盐是200°C时形成的主要结构。在较低温度下,会形成硝酸盐螯合和单齿硝酸盐的混合物,以及硝态氮离子NO +,其含量与布朗斯台德沸石的浓度成正比。发现硝酸盐主要由Cu II离子形成,该Cu II离子由一种负构架电荷(Z),Z- [Cu(OH] I或Z- [Cu(O 2 ] I)稳定,而没有Z的参与2铜II的。这一证据,加上样品中没有高架铜-铜对的硝酸盐桥接现象,表明硝酸盐配体不能迁移铜离子,这与最近报道的NH 3有所不同。最后,发现在两个温度下水都可以代替预先形成的螯合硝酸铜,并消耗NO +(尽管动力学不同),同时有利于单齿的存在。
更新日期:2018-07-26
中文翻译:

研究Cu-CHA沸石上CuII-(N,O)物种的低温形成,以选择性催化还原NOx
在这项工作中,我们显示operando FTIR的潜力光谱分析遵循形成Cu II - (N,O)对Cu物种交换的菱沸石的沸石法(Cu-CHA),活性为选择性催化还原NO的X与NH 3( NH 3 -SCR)。特别是,我们研究了NO和O 2的反应在低温下(200和50°C)在一系列具有不同成分(Si / Al和Cu / Al比)的Cu-CHA沸石上研究形成的硝酸铜的性质,这些硝酸铜被认为是关键的中间体在SCR循环的氧化部分。我们的结果表明,螯合的双齿硝酸盐是200°C时形成的主要结构。在较低温度下,会形成硝酸盐螯合和单齿硝酸盐的混合物,以及硝态氮离子NO +,其含量与布朗斯台德沸石的浓度成正比。发现硝酸盐主要由Cu II离子形成,该Cu II离子由一种负构架电荷(Z),Z- [Cu(OH] I或Z- [Cu(O 2 ] I)稳定,而没有Z的参与2铜II的。这一证据,加上样品中没有高架铜-铜对的硝酸盐桥接现象,表明硝酸盐配体不能迁移铜离子,这与最近报道的NH 3有所不同。最后,发现在两个温度下水都可以代替预先形成的螯合硝酸铜,并消耗NO +(尽管动力学不同),同时有利于单齿的存在。



























 京公网安备 11010802027423号
京公网安备 11010802027423号