当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
[3,3]‐Sigmatropic Rearrangement of Cyclopropenylcarbinyl Cyanates: Access to Alkylidene(aminocyclopropane) Derivatives
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-09-10 , DOI: 10.1002/chem.201803231 Guillaume Ernouf 1 , Jean-Louis Brayer 2 , Benoît Folléas 2 , Jean-Pierre Demoute 2 , Christophe Meyer 1 , Janine Cossy 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-09-10 , DOI: 10.1002/chem.201803231 Guillaume Ernouf 1 , Jean-Louis Brayer 2 , Benoît Folléas 2 , Jean-Pierre Demoute 2 , Christophe Meyer 1 , Janine Cossy 1
Affiliation
|
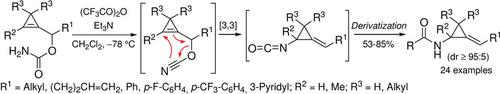
Cyclopropenylcarbinyl cyanates, generated in situ by dehydration of the corresponding carbamates, underwent an efficient and stereoselective [3,3]‐sigmatropic rearrangement leading to the corresponding alkylidene(isocyanatocyclopropanes), which could be converted into various alkylidene(aminocyclopropane) derivatives in a one‐pot manner. This transformation complements the repertoire of sigmatropic rearrangements involving cyclopropenylcarbinol derivatives and in particular, the previously reported Overman rearrangement of cyclopropenylcarbinyl trichloroacetimidates.
中文翻译:

[3,3]-环丙烯基羰基氰基酸酯的正向重排:获得亚炔基(氨基环丙烷)衍生物
通过相应的氨基甲酸酯脱水原位生成的环丙烯基羰基氰酸酯,经过高效且立体选择性的[3,3]-σ重排,导致相应的亚烷基(异氰酸根合环丙烷),可以将其转化为多种亚烷基(氨基环丙烷)衍生物。锅方式。这种转变补充了涉及环丙烯基甲醇衍生物的σ重排的所有组成,特别是先前报道的环丙烯基羰基三氯乙酰亚胺酸酯的超人重排。
更新日期:2018-09-10
中文翻译:

[3,3]-环丙烯基羰基氰基酸酯的正向重排:获得亚炔基(氨基环丙烷)衍生物
通过相应的氨基甲酸酯脱水原位生成的环丙烯基羰基氰酸酯,经过高效且立体选择性的[3,3]-σ重排,导致相应的亚烷基(异氰酸根合环丙烷),可以将其转化为多种亚烷基(氨基环丙烷)衍生物。锅方式。这种转变补充了涉及环丙烯基甲醇衍生物的σ重排的所有组成,特别是先前报道的环丙烯基羰基三氯乙酰亚胺酸酯的超人重排。











































 京公网安备 11010802027423号
京公网安备 11010802027423号