当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Building Molecular Complexity from Quinizarin: Conjoined Coumarins and Coronene Analogs
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2018-08-27 , DOI: 10.1002/asia.201800757 Marek K. Węcławski 1 , Irena Deperasińska 2 , Marzena Banasiewicz 2 , David C. Young 1 , Arkadiusz Leniak 1 , Daniel T. Gryko 1
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2018-08-27 , DOI: 10.1002/asia.201800757 Marek K. Węcławski 1 , Irena Deperasińska 2 , Marzena Banasiewicz 2 , David C. Young 1 , Arkadiusz Leniak 1 , Daniel T. Gryko 1
Affiliation
|
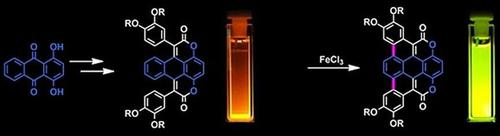
The double Knoevenagel condensation of 1,4‐dibenzoyloxyanthraquinone with methyl esters of arylacetic acids affords a series of compounds based upon a previously unknown 1,8‐dioxa‐benzo[e]pyrene‐2,7‐dione heterocyclic core. The aryl groups incorporated in the 3‐ and 6‐positions can be oxidatively coupled to the π‐expanded backbone to produce a further new heterocyclic core: 1,10‐dioxa‐dibenzo[dj]coronene‐2,9‐dione. The intriguing optical properties of these π‐expanded coumarin derivatives are discussed and rationalized through quantum chemical calculations. The broad absorption bands of 1,8‐dioxa‐benzo[e]pyrene‐2,7‐dione‐based dyes are attributed to both HOMO−1→LUMO and HOMO→LUMO transitions, which have a similar energy. Weakly coupled electron‐donating aryl substituents result in a moderate bathochromic shift of both the absorption and emission by 30–60 nm in toluene. The emissive properties of these compounds are in part determined by the oscillator strength of the main transition, lifetimes of the excited state, and by the energy match of the excited state with a triplet state of a similar energy. The 1,10‐dioxa‐dibenzo[dj]coronene‐2,9‐dione displays a much smaller Stokes shift, yet a markedly increased fluorescence quantum yield of 90 % owing to the increased rigidity compared with the 1,8‐dioxa‐benzo[e]pyrene‐2,7‐dione core.
中文翻译:

从奎尼萨林提高分子的复杂性:香豆素和类似物的类似物
1,4-二苯甲酰氧基蒽醌与芳酸甲酯的双Knoevenagel缩合反应提供了一系列基于以前未知的1,8-二氧杂苯并[ e ] py-2,7-二酮杂环核的化合物。结合在3位和6位上的芳基可以与π扩展的骨架进行氧化偶联,以产生另一个新的杂环核:1,10-dioxa-dibenzo [ dj ] coronene-2,9-dione。通过量子化学计算讨论并合理化了这些π扩展香豆素衍生物的引人入胜的光学性质。1,8-二氧杂苯并[ e]的宽吸收带] pyrene-2,7-二酮基染料归因于HOMO-1→LUMO和HOMO→LUMO跃迁,它们具有相似的能量。弱耦合的给电子芳基取代基导致甲苯吸收和发射发生适度的红移30-60 nm。这些化合物的发射特性部分取决于主跃迁的振子强度,受激态的寿命以及受激态与类似能量的三重态的能量匹配。1,10-二氧杂二苯并[ dj ]二甲苯_2,9-二酮的斯托克斯位移小得多,但与1,8-二氧杂苯并benzo相比,由于刚性提高,荧光量子产率显着提高了90% [ e ] pyrene-2,7-dione核心。
更新日期:2018-08-27
中文翻译:

从奎尼萨林提高分子的复杂性:香豆素和类似物的类似物
1,4-二苯甲酰氧基蒽醌与芳酸甲酯的双Knoevenagel缩合反应提供了一系列基于以前未知的1,8-二氧杂苯并[ e ] py-2,7-二酮杂环核的化合物。结合在3位和6位上的芳基可以与π扩展的骨架进行氧化偶联,以产生另一个新的杂环核:1,10-dioxa-dibenzo [ dj ] coronene-2,9-dione。通过量子化学计算讨论并合理化了这些π扩展香豆素衍生物的引人入胜的光学性质。1,8-二氧杂苯并[ e]的宽吸收带] pyrene-2,7-二酮基染料归因于HOMO-1→LUMO和HOMO→LUMO跃迁,它们具有相似的能量。弱耦合的给电子芳基取代基导致甲苯吸收和发射发生适度的红移30-60 nm。这些化合物的发射特性部分取决于主跃迁的振子强度,受激态的寿命以及受激态与类似能量的三重态的能量匹配。1,10-二氧杂二苯并[ dj ]二甲苯_2,9-二酮的斯托克斯位移小得多,但与1,8-二氧杂苯并benzo相比,由于刚性提高,荧光量子产率显着提高了90% [ e ] pyrene-2,7-dione核心。











































 京公网安备 11010802027423号
京公网安备 11010802027423号