当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights into Interfacial Synergistic Catalysis over Ni@TiO2-x Catalyst toward Water-Gas Shift Reaction
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-07-17 , DOI: 10.1021/jacs.8b03117 Ming Xu 1 , Siyu Yao 2 , Deming Rao 1 , Yiming Niu 3 , Ning Liu 1 , Mi Peng 2 , Peng Zhai 2 , Yi Man 4 , Lirong Zheng 5 , Bin Wang 4 , Bingsen Zhang 3 , Ding Ma 2 , Min Wei 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-07-17 , DOI: 10.1021/jacs.8b03117 Ming Xu 1 , Siyu Yao 2 , Deming Rao 1 , Yiming Niu 3 , Ning Liu 1 , Mi Peng 2 , Peng Zhai 2 , Yi Man 4 , Lirong Zheng 5 , Bin Wang 4 , Bingsen Zhang 3 , Ding Ma 2 , Min Wei 1
Affiliation
|
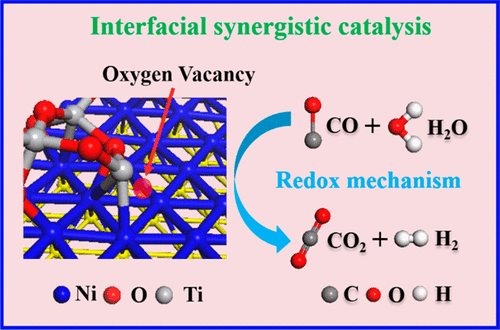
The mechanism on interfacial synergistic catalysis for supported metal catalysts has long been explored and investigated in several important heterogeneous catalytic processes (e.g., water-gas shift (WGS) reaction). The modulation of metal-support interactions imposes a substantial influence on activity and selectivity of catalytic reaction, as a result of the geometric/electronic structure of interfacial sites. Although great efforts have validated the key role of interfacial sites in WGS over metal catalysts supported on reducible oxides, direct evidence at the atomic level is lacking and the mechanism of interfacial synergistic catalysis is still ambiguous. Herein, Ni nanoparticles supported on TiO2- x (denoted as Ni@TiO2- x) were fabricated via a structure topotactic transformation of NiTi-layered double hydroxide (NiTi-LDHs) precursor, which showed excellent catalytic performance for WGS reaction. In situ microscopy was carried out to reveal the partially encapsulated structure of Ni@TiO2- x catalyst. A combination study including in situ and operando EXAFS, in situ DRIFTS spectra combined with TPSR measurements substantiates a new redox mechanism based on interfacial synergistic catalysis. Notably, interfacial Ni species (electron-enriched Niδ- site) participates in the dissociation of H2O molecule to generate H2, accompanied by the oxidation of Niδ--O v-Ti3+ (O v: oxygen vacancy) to Niδ+-O-Ti4+ structure. Density functional theory calculations further verify that the interfacial sites of Ni@TiO2- x catalyst serve as the optimal active site with the lowest activation energy barrier (∼0.35 eV) for water dissociation. This work provides a fundamental understanding on interfacial synergistic catalysis toward WGS reaction, which is constructive for the rational design and fabrication of high activity heterogeneous catalysts.
中文翻译:

Ni@TiO2-x 催化剂界面协同催化水煤气变换反应的洞察
长期以来,在几个重要的多相催化过程(例如,水煤气变换(WGS)反应)中,一直在探索和研究负载金属催化剂的界面协同催化机制。由于界面位点的几何/电子结构,金属-载体相互作用的调节对催化反应的活性和选择性产生重大影响。尽管大量的努力已经验证了界面位点在可还原氧化物负载的金属催化剂中的关键作用,但缺乏原子水平的直接证据,界面协同催化的机制仍然不明确。在此,通过 NiTi 层状双氢氧化物 (NiTi-LDHs) 前驱体的结构拓扑转化制备了负载在 TiO2-x 上的 Ni 纳米颗粒(表示为 Ni@TiO2-x),对WGS反应表现出优异的催化性能。进行原位显微镜检查以揭示 Ni@TiO2-x 催化剂的部分包封结构。包括原位和原位 EXAFS、原位 DRIFTS 光谱与 TPSR 测量相结合的组合研究证实了一种基于界面协同催化的新氧化还原机制。值得注意的是,界面 Ni 物种(富电子 Niδ-位点)参与 H2O 分子的解离以生成 H2,伴随着 Niδ--O v-Ti3+(O v:氧空位)氧化为 Niδ+-O-Ti4+结构体。密度泛函理论计算进一步验证了 Ni@TiO2-x 催化剂的界面位点作为最佳活性位点,具有最低的水离解活化能垒(~0.35 eV)。
更新日期:2018-07-17
中文翻译:

Ni@TiO2-x 催化剂界面协同催化水煤气变换反应的洞察
长期以来,在几个重要的多相催化过程(例如,水煤气变换(WGS)反应)中,一直在探索和研究负载金属催化剂的界面协同催化机制。由于界面位点的几何/电子结构,金属-载体相互作用的调节对催化反应的活性和选择性产生重大影响。尽管大量的努力已经验证了界面位点在可还原氧化物负载的金属催化剂中的关键作用,但缺乏原子水平的直接证据,界面协同催化的机制仍然不明确。在此,通过 NiTi 层状双氢氧化物 (NiTi-LDHs) 前驱体的结构拓扑转化制备了负载在 TiO2-x 上的 Ni 纳米颗粒(表示为 Ni@TiO2-x),对WGS反应表现出优异的催化性能。进行原位显微镜检查以揭示 Ni@TiO2-x 催化剂的部分包封结构。包括原位和原位 EXAFS、原位 DRIFTS 光谱与 TPSR 测量相结合的组合研究证实了一种基于界面协同催化的新氧化还原机制。值得注意的是,界面 Ni 物种(富电子 Niδ-位点)参与 H2O 分子的解离以生成 H2,伴随着 Niδ--O v-Ti3+(O v:氧空位)氧化为 Niδ+-O-Ti4+结构体。密度泛函理论计算进一步验证了 Ni@TiO2-x 催化剂的界面位点作为最佳活性位点,具有最低的水离解活化能垒(~0.35 eV)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号