当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Monomeric Rare‐Earth Metal Silyl‐Thiophosphinoyl‐Alkylidene Complexes: Synthesis, Structure, and Reactivity
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-08-22 , DOI: 10.1002/chem.201802791 Chen Wang 1 , Weiqing Mao 1 , Li Xiang 1 , Yan Yang 2 , Jian Fang 2 , Laurent Maron 3 , Xuebing Leng 1 , Yaofeng Chen 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-08-22 , DOI: 10.1002/chem.201802791 Chen Wang 1 , Weiqing Mao 1 , Li Xiang 1 , Yan Yang 2 , Jian Fang 2 , Laurent Maron 3 , Xuebing Leng 1 , Yaofeng Chen 1
Affiliation
|
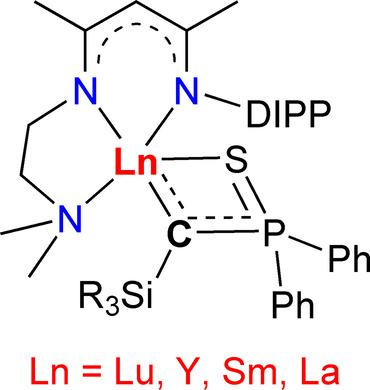
A series of monomeric rare‐earth metal silyl‐thiophosphinoyl‐alkylidene complexes [LLn{C(SiR3)PPh2S}] (5: Ln=Lu, R=Me; 6: Ln=Lu, R=Ph; 7: Ln=Y, R=Me; 8: Ln=Y, R=Ph; 9: Ln=Sm, R=Ph; 10: Ln=Sm, R=Me; 11: Ln=La, R=Ph; L=[MeC(NDIPP)CHC(Me)(NCH2CH2N(Me)2)]−, DIPP=2,6‐(iPr)2C6H3) have been synthesized and structurally characterized. The influences of rare‐earth metal ions, ancillary ligands, and alkylidene groups on the reactivity of complexes 5–11 and the related scandium complexes [LSc{C(SiR3)PPh2S}] (1: R=Me; 2: R=Ph) and [L′Sc{C(SiR3)PPh2S}] (3: R=Me; 4: R=Ph; L′=[MeC(NDIPP)CHC(Me)(NCH2CH2N(iPr)2)]−) have been studied. Reactions of these rare‐earth metal alkylidene complexes with PhCN give four kinds of products, the formation of which is dependent on the rare‐earth metal ions, ancillary ligands, and alkylidene groups of the complexes. In the reactions with tBuNC, unusual C−P bond cleavage of the alkylidene group and C≡C triple bond formation occur. Complexes 10 and 11 also react with PhSiH3 to form hydrides, which subsequently undergo Ln−H addition to the C=N bond of the ancillary ligand L. DFT calculations have been used to analyze the bonding in complex 10, which exhibits a polarized three centers Sm−C−P π interaction, and to rationalize the reactivity by computing reaction mechanisms. The difference in reactivity of PhCN and tBuNC is due to the electron density delocalization that is enabled by the phenyl group rather than the tBu group.
中文翻译:

单体稀土金属甲硅烷基-硫代膦酰基-亚炔基配合物:合成,结构和反应性
一系列单体稀土金属硅烷基-硫代膦酰基-亚烷基络合物[LLn {C(SiR 3)PPh 2 S}](5:Ln = Lu,R = Me; 6:Ln = Lu,R = Ph; 7: Ln = Y,R = Me; 8:Ln = Y,R = Ph; 9:Ln = Sm,R = Ph; 10:Ln = Sm,R = Me; 11:Ln = La,R = Ph; L = [MeC(NDIPP)CHC(Me)(NCH 2 CH 2 N(Me)2)] -,DIPP = 2.6-(i Pr)2 C 6 H 3)已合成并进行了结构表征。稀土类金属离子,辅助配体,和亚烷基的上的复合物的反应性的影响5 - 11和相关的钪络合物[LSC {C(SIR 3)PPH 2 S}](1:R =甲基; 2: R = Ph)和[L'Sc {C(SiR 3)PPh 2 S}](3:R = Me; 4:R = Ph; L'= [MeC(NDIPP)CHC(Me)(NCH 2 CH 2 N(i Pr)2)] -)已进行了研究。这些稀土金属亚烷基配合物与PhCN的反应产生四种产物,其形成取决于配合物的稀土金属离子,辅助配体和亚烷基。在与t BuNC的反应中,发生亚烷基的不寻常的C-P键断裂和C≡C三键形成。配合物10和11也与PhSiH 3反应形成氢化物,随后氢化物经过Ln-H加到辅助配体L的C = N键上。DFT计算已用于分析配合物10中的键合,它表现出极化的三个中心Sm-C-Pπ相互作用,并且通过计算反应机理来合理化反应性。PhCN和t BuNC的反应性差异是由于苯基而不是t Bu基团引起的电子密度离域。
更新日期:2018-08-22
中文翻译:

单体稀土金属甲硅烷基-硫代膦酰基-亚炔基配合物:合成,结构和反应性
一系列单体稀土金属硅烷基-硫代膦酰基-亚烷基络合物[LLn {C(SiR 3)PPh 2 S}](5:Ln = Lu,R = Me; 6:Ln = Lu,R = Ph; 7: Ln = Y,R = Me; 8:Ln = Y,R = Ph; 9:Ln = Sm,R = Ph; 10:Ln = Sm,R = Me; 11:Ln = La,R = Ph; L = [MeC(NDIPP)CHC(Me)(NCH 2 CH 2 N(Me)2)] -,DIPP = 2.6-(i Pr)2 C 6 H 3)已合成并进行了结构表征。稀土类金属离子,辅助配体,和亚烷基的上的复合物的反应性的影响5 - 11和相关的钪络合物[LSC {C(SIR 3)PPH 2 S}](1:R =甲基; 2: R = Ph)和[L'Sc {C(SiR 3)PPh 2 S}](3:R = Me; 4:R = Ph; L'= [MeC(NDIPP)CHC(Me)(NCH 2 CH 2 N(i Pr)2)] -)已进行了研究。这些稀土金属亚烷基配合物与PhCN的反应产生四种产物,其形成取决于配合物的稀土金属离子,辅助配体和亚烷基。在与t BuNC的反应中,发生亚烷基的不寻常的C-P键断裂和C≡C三键形成。配合物10和11也与PhSiH 3反应形成氢化物,随后氢化物经过Ln-H加到辅助配体L的C = N键上。DFT计算已用于分析配合物10中的键合,它表现出极化的三个中心Sm-C-Pπ相互作用,并且通过计算反应机理来合理化反应性。PhCN和t BuNC的反应性差异是由于苯基而不是t Bu基团引起的电子密度离域。











































 京公网安备 11010802027423号
京公网安备 11010802027423号