当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diisopropylethylamine-triggered, highly efficient, self-catalyzed regioselective acylation of carbohydrates and diols†
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-07-18 00:00:00 , DOI: 10.1039/c8ob01464g Bo Ren 1, 2, 3, 4 , Lu Gan 1, 2, 3, 4 , Li Zhang 1, 2, 3, 4 , Ningning Yan 1, 2, 3, 4 , Hai Dong 4, 5, 6, 7
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-07-18 00:00:00 , DOI: 10.1039/c8ob01464g Bo Ren 1, 2, 3, 4 , Lu Gan 1, 2, 3, 4 , Li Zhang 1, 2, 3, 4 , Ningning Yan 1, 2, 3, 4 , Hai Dong 4, 5, 6, 7
Affiliation
|
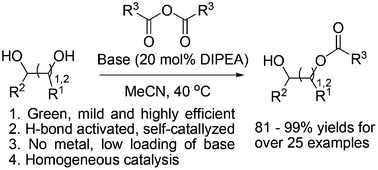
A diisopropylethylamine (DIPEA)-triggered, self-catalyzed, regioselective acylation of carbohydrates and diols is presented. The hydroxyl groups can be acylated by the corresponding anhydride in MeCN in the presence of a catalytic amount of DIPEA. This method is comparatively green and mild as it uses less organic base compared with other selective acylation methods. Mechanistic studies indicate that DIPEA reacts with the anhydride to form a carboxylate ion, and then the carboxylate ion could catalyze the selective acylation through a dual H-bonding interaction.
中文翻译:

碳水化合物和二醇的二异丙基乙胺触发的高效自催化区域选择性酰化†
提出了二异丙基乙胺(DIPEA)引发的碳水化合物和二醇的自催化区域选择性酰化反应。羟基可以在催化量的DIPEA存在下被MeCN中的相应酸酐酰化。与其他选择性酰化方法相比,该方法使用的有机碱较少,因此相对绿色且温和。机理研究表明,DIPEA与酸酐反应形成羧酸根离子,然后羧酸根离子可以通过双重氢键相互作用催化选择性酰化反应。
更新日期:2018-07-18
中文翻译:

碳水化合物和二醇的二异丙基乙胺触发的高效自催化区域选择性酰化†
提出了二异丙基乙胺(DIPEA)引发的碳水化合物和二醇的自催化区域选择性酰化反应。羟基可以在催化量的DIPEA存在下被MeCN中的相应酸酐酰化。与其他选择性酰化方法相比,该方法使用的有机碱较少,因此相对绿色且温和。机理研究表明,DIPEA与酸酐反应形成羧酸根离子,然后羧酸根离子可以通过双重氢键相互作用催化选择性酰化反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号