当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Brain Targeting by Liposome-Biomolecular Corona Boosts Anticancer Efficacy of Temozolomide in Glioblastoma Cells.
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-07-31 , DOI: 10.1021/acschemneuro.8b00339 Antonietta Arcella 1 , Sara Palchetti 2 , Luca Digiacomo 2 , Daniela Pozzi 2 , Anna Laura Capriotti 3 , Luigi Frati 1 , Maria Antonietta Oliva 1 , Georgia Tsaouli 2 , Rossella Rota 4 , Isabella Screpanti 2 , Morteza Mahmoudi 5 , Giulio Caracciolo 2
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-07-31 , DOI: 10.1021/acschemneuro.8b00339 Antonietta Arcella 1 , Sara Palchetti 2 , Luca Digiacomo 2 , Daniela Pozzi 2 , Anna Laura Capriotti 3 , Luigi Frati 1 , Maria Antonietta Oliva 1 , Georgia Tsaouli 2 , Rossella Rota 4 , Isabella Screpanti 2 , Morteza Mahmoudi 5 , Giulio Caracciolo 2
Affiliation
|
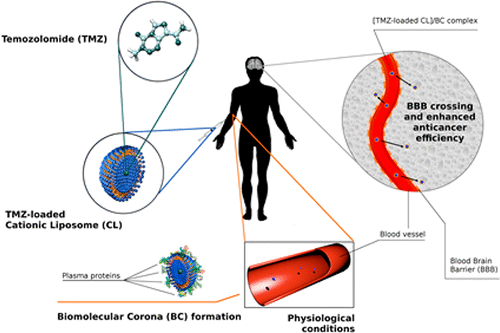
Temozolomide (TMZ) is the current first-line chemotherapy for treatment of glioblastoma multiforme (GBM). However, similar to other brain therapeutic compounds, access of TMZ to brain tumors is impaired by the blood-brain barrier (BBB) leading to poor response for GBM patients. To overcome this major hurdle, we have synthesized a set of TMZ-encapsulating nanomedicines made of four cationic liposome (CL) formulations with systematic changes in lipid composition and physical-chemical properties. The targeting nature of this nanomedicine is provided by the recruitment of proteins, with natural targeting capacity, in the biomolecular corona (BC) layer that forms around CLs after exposure to human plasma (HP). TMZ-loaded CL-BC complexes were thoroughly characterized by dynamic light scattering (DLS), electrophoretic light scattering (ELS), and nanoliquid chromatography tandem mass spectrometry (nano-LC MS/MS). BCs were found to be enriched of typical BC fingerprints (BCFs) (e.g., Apolipoproteins, Vitronectin, and vitamin K-dependent protein), which have a substantial capacity in binding to receptors that are overexpressed at the BBB (e.g., scavenger receptor class B, type I and low-density lipoprotein receptor). We found that the CL formulation exhibiting the highest levels of targeting BCFs had larger uptake in human umbilical vein endothelial cells (HUVECs) that are commonly used as an in vitro model of the BBB. This formulation could also deliver TMZ to the human glioblastoma U-87 MG cell line and thus substantially enhance their antitumor efficacy compared to corona free CLs. Thus, we propose that the BC-based nanomedicines may pave a more effective way for efficient treatment of GBM.
中文翻译:

脂质体-生物分子电晕对大脑的靶向作用增强了替莫唑胺在成胶质细胞瘤细胞中的抗癌功效。
替莫唑胺(TMZ)是目前治疗多形性胶质母细胞瘤(GBM)的一线化疗。但是,类似于其他脑部治疗化合物,血脑屏障(BBB)会损害TMZ进入脑肿瘤的能力,从而导致GBM患者的不良反应。为了克服这一主要障碍,我们合成了一组由四种阳离子脂质体(CL)制剂制成的TMZ封装纳米药物,这些制剂在脂质组成和物理化学性质上都有系统性的变化。这种纳米药物的靶向性质是通过在自然血浆中暴露于人类血浆(HP)后在CL周围形成的生物分子电晕(BC)层中募集具有自然靶向能力的蛋白质来实现的。负载TMZ的CL-BC复合物通过动态光散射(DLS),电泳光散射(ELS),和纳米液相色谱串联质谱法(纳米LC MS / MS)。发现BC富含典型的BC指纹(BCF)(例如载脂蛋白,玻连蛋白和维生素K依赖性蛋白),它们具有与BBB过表达的受体(例如,清道夫受体B类)结合的显着能力。 ,I型和低密度脂蛋白受体)。我们发现,具有最高水平的靶向BCF的CL制剂在通常用作BBB体外模型的人脐静脉内皮细胞(HUVEC)中具有更大的摄取量。该制剂还可以将TMZ递送至人胶质母细胞瘤U-87 MG细胞系,因此与不含电晕的CL相比,其显着增强了其抗肿瘤功效。因此,
更新日期:2018-07-17
中文翻译:

脂质体-生物分子电晕对大脑的靶向作用增强了替莫唑胺在成胶质细胞瘤细胞中的抗癌功效。
替莫唑胺(TMZ)是目前治疗多形性胶质母细胞瘤(GBM)的一线化疗。但是,类似于其他脑部治疗化合物,血脑屏障(BBB)会损害TMZ进入脑肿瘤的能力,从而导致GBM患者的不良反应。为了克服这一主要障碍,我们合成了一组由四种阳离子脂质体(CL)制剂制成的TMZ封装纳米药物,这些制剂在脂质组成和物理化学性质上都有系统性的变化。这种纳米药物的靶向性质是通过在自然血浆中暴露于人类血浆(HP)后在CL周围形成的生物分子电晕(BC)层中募集具有自然靶向能力的蛋白质来实现的。负载TMZ的CL-BC复合物通过动态光散射(DLS),电泳光散射(ELS),和纳米液相色谱串联质谱法(纳米LC MS / MS)。发现BC富含典型的BC指纹(BCF)(例如载脂蛋白,玻连蛋白和维生素K依赖性蛋白),它们具有与BBB过表达的受体(例如,清道夫受体B类)结合的显着能力。 ,I型和低密度脂蛋白受体)。我们发现,具有最高水平的靶向BCF的CL制剂在通常用作BBB体外模型的人脐静脉内皮细胞(HUVEC)中具有更大的摄取量。该制剂还可以将TMZ递送至人胶质母细胞瘤U-87 MG细胞系,因此与不含电晕的CL相比,其显着增强了其抗肿瘤功效。因此,











































 京公网安备 11010802027423号
京公网安备 11010802027423号