当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facile incorporation of technetium into magnetite, magnesioferrite, and hematite by formation of ferrous nitrate in situ: precursors to iron oxide nuclear waste forms
Dalton Transactions ( IF 3.5 ) Pub Date : 2018-07-17 00:00:00 , DOI: 10.1039/c8dt01356j Wayne W. Lukens 1, 2, 3, 4 , Sarah A. Saslow 4, 5, 6, 7
Dalton Transactions ( IF 3.5 ) Pub Date : 2018-07-17 00:00:00 , DOI: 10.1039/c8dt01356j Wayne W. Lukens 1, 2, 3, 4 , Sarah A. Saslow 4, 5, 6, 7
Affiliation
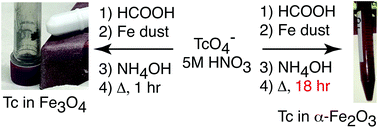
|
The fission product, 99Tc, presents significant challenges to the long-term disposal of nuclear waste due to its long half-life, high fission yield, and to the environmental mobility of pertechnetate (TcO4−), the stable Tc species in aerobic environments. Migration of 99Tc from disposal sites can potentially be prevented by incorporating it into durable waste forms based on environmentally stable minerals. Since Tc(IV) and Fe(III) have the same ionic radius, Tc(IV) can replace Fe(III) in iron oxides. Environmentally durable iron oxides include goethite (α-FeOOH), hematite (α-Fe2O3), and magnesioferrite (MgFe2O4). The incorporation of Tc into two of these, hematite and magnesioferrite, as well as magnetite (Fe3O4) by means of simple, aqueous chemistry is presented starting from TcO4− in 5 M nitric acid. A combination of X-ray diffraction and X-ray absorption fine structure spectroscopy reveals that Tc(IV) replaces Fe(III) within the iron oxide structures. Following incorporation, Tc doped samples were suspended in deionized water under aerobic conditions, and the release rates of Tc were determined. The results of this work show that Tc leaches more quickly from Fe3O4 than from α-Fe2O3 or MgFe2O4. Modeling the leach rates and comparison with the leach rate of Tc from TiO2 indicate that release of Tc is controlled by solid state diffusion.
中文翻译:

通过就地形成硝酸亚铁将tech容易地掺入磁铁矿,镁铁矿和赤铁矿中:氧化铁核废料形式的前体
裂变产物99 Tc,由于其半衰期长,裂变收率高以及高tech酸盐(TcO 4 -)(好氧条件下稳定的Tc物种)的环境迁移性,对核废料的长期处置提出了重大挑战。环境。通过将99 Tc掺入基于环境稳定矿物的持久废物中,可以潜在地防止其从处置场所迁移。由于Tc(IV)和Fe(III)具有相同的离子半径,因此Tc(IV)可以代替氧化铁中的Fe(III)。环境耐用铁氧化物包括针铁矿(α-的FeOOH),赤铁矿(α-的Fe 2 ö 3)和镁铁氧体(MgFe 2 O 4)。Tc的掺入两种这些,赤铁矿和magnesioferrite的,以及磁铁矿(铁3 ö 4通过简单的手段),含水化学呈现从TCO开始4 -在5M的硝酸。X射线衍射和X射线吸收精细结构光谱的组合显示,Tc(IV)代替了铁氧化物结构中的Fe(III)。掺入后,将掺有Tc的样品在有氧条件下悬浮在去离子水中,并测定Tc的释放速率。这项工作的结果表明,Tc从Fe 3 O 4中浸出的速度更快。比从的α-Fe 2 ö 3或MgFe 2 Ò 4。对浸出速率进行建模并与TiO 2中Tc的浸出速率进行比较表明,Tc的释放受固态扩散的控制。
更新日期:2018-07-17
中文翻译:

通过就地形成硝酸亚铁将tech容易地掺入磁铁矿,镁铁矿和赤铁矿中:氧化铁核废料形式的前体
裂变产物99 Tc,由于其半衰期长,裂变收率高以及高tech酸盐(TcO 4 -)(好氧条件下稳定的Tc物种)的环境迁移性,对核废料的长期处置提出了重大挑战。环境。通过将99 Tc掺入基于环境稳定矿物的持久废物中,可以潜在地防止其从处置场所迁移。由于Tc(IV)和Fe(III)具有相同的离子半径,因此Tc(IV)可以代替氧化铁中的Fe(III)。环境耐用铁氧化物包括针铁矿(α-的FeOOH),赤铁矿(α-的Fe 2 ö 3)和镁铁氧体(MgFe 2 O 4)。Tc的掺入两种这些,赤铁矿和magnesioferrite的,以及磁铁矿(铁3 ö 4通过简单的手段),含水化学呈现从TCO开始4 -在5M的硝酸。X射线衍射和X射线吸收精细结构光谱的组合显示,Tc(IV)代替了铁氧化物结构中的Fe(III)。掺入后,将掺有Tc的样品在有氧条件下悬浮在去离子水中,并测定Tc的释放速率。这项工作的结果表明,Tc从Fe 3 O 4中浸出的速度更快。比从的α-Fe 2 ö 3或MgFe 2 Ò 4。对浸出速率进行建模并与TiO 2中Tc的浸出速率进行比较表明,Tc的释放受固态扩散的控制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号