Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Behavior of Single CuO Nanoparticles: Implications for the Assessment of their Environmental Fate
Small ( IF 13.0 ) Pub Date : 2018-07-17 , DOI: 10.1002/smll.201801765 Giorgia Zampardi 1 , Jorg Thöming 2, 3 , Hendrik Naatz 3, 4 , Hatem M. A. Amin 1 , Suman Pokhrel 3, 4 , Lutz Mädler 3, 4 , Richard G. Compton 1
Small ( IF 13.0 ) Pub Date : 2018-07-17 , DOI: 10.1002/smll.201801765 Giorgia Zampardi 1 , Jorg Thöming 2, 3 , Hendrik Naatz 3, 4 , Hatem M. A. Amin 1 , Suman Pokhrel 3, 4 , Lutz Mädler 3, 4 , Richard G. Compton 1
Affiliation
|
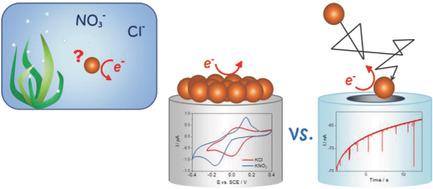
The electrochemical behavior of copper oxide nanoparticles is investigated at both the single particle and at the ensemble level in neutral aqueous solutions through the electrode‐particle collision method and cyclic voltammetry, respectively. The influence of Cl− and NO3− anions on the electrochemical processes occurring at the nanoparticles is further evaluated. The electroactivity of CuO nanoparticles is found to differ between the two types of experiments. At the single‐particle scale, the reduction of the CuO nanoparticles proceeds to a higher extent in the presence of chloride ion than of nitrate ion containing solutions. However, at the multiparticle scale the CuO reduction proceeds to the same extent regardless of the type of anions present in solution. The implications for assessing realistically the environmental fate and therefore the toxicity of metal‐based nanoparticles in general, and copper‐based nanoparticles in particular, are discussed.
中文翻译:

单个CuO纳米粒子的电化学行为:对评估其环境命运的启示
分别通过电极-颗粒碰撞法和循环伏安法研究了中性水溶液中铜氧化物纳米粒子的电化学行为,包括在单个粒子上和在集合级上的行为。氯的影响-和NO 3 -进一步评估了纳米颗粒上发生的电化学过程中的阴离子。发现在两种类型的实验之间,CuO纳米粒子的电活性不同。在单颗粒规模上,在存在氯离子的情况下,与含硝酸盐溶液相比,CuO纳米颗粒的还原程度更高。但是,无论溶液中存在的阴离子类型如何,在多粒子规模下,CuO还原的程度都相同。讨论了评估现实环境命运的意义,进而评估了金属基纳米颗粒,特别是铜基纳米颗粒的毒性。
更新日期:2018-07-17
中文翻译:

单个CuO纳米粒子的电化学行为:对评估其环境命运的启示
分别通过电极-颗粒碰撞法和循环伏安法研究了中性水溶液中铜氧化物纳米粒子的电化学行为,包括在单个粒子上和在集合级上的行为。氯的影响-和NO 3 -进一步评估了纳米颗粒上发生的电化学过程中的阴离子。发现在两种类型的实验之间,CuO纳米粒子的电活性不同。在单颗粒规模上,在存在氯离子的情况下,与含硝酸盐溶液相比,CuO纳米颗粒的还原程度更高。但是,无论溶液中存在的阴离子类型如何,在多粒子规模下,CuO还原的程度都相同。讨论了评估现实环境命运的意义,进而评估了金属基纳米颗粒,特别是铜基纳米颗粒的毒性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号