当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Analogues of Arylamide Phenylpiperazine Ligands To Investigate the Factors Influencing D3 Dopamine Receptor Bitropic Binding and Receptor Subtype Selectivity.
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-07-30 , DOI: 10.1021/acschemneuro.8b00142 Hamed S Hayatshahi 1 , Kuiying Xu 2 , Suzy A Griffin 3 , Michelle Taylor 3 , Robert H Mach 2 , Jin Liu 1 , Robert R Luedtke 3
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-07-30 , DOI: 10.1021/acschemneuro.8b00142 Hamed S Hayatshahi 1 , Kuiying Xu 2 , Suzy A Griffin 3 , Michelle Taylor 3 , Robert H Mach 2 , Jin Liu 1 , Robert R Luedtke 3
Affiliation
|
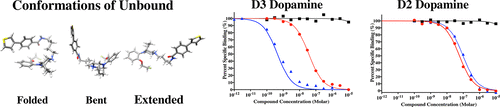
We have previously reported on the ability of arylamide phenylpiperazines to bind selectively to the D3 versus the D2 dopamine receptor subtype. For these studies, we used LS-3-134 as the prototypic arylamide phenylpiperazine ligand because it binds with high affinity at D3 dopamine receptor (0.17 nM) and exhibits >150-fold D3 vs D2 receptor binding selectivity. Our goal was to investigate how the composition and size of the nonaromatic ring structure at the piperazine position of substituted phenylpiperazine analogues might influence binding affinity at the human D2 and D3 dopamine receptors. Two factors were identified as being important for determining the binding affinity of bitropic arylamide phenylpiperazines at the dopamine D3 receptor subtype. One factor was the strength of the salt bridge between the highly conserved residue Asp3.32 with the protonated nitrogen of the nonaromatic ring at the piperazine position. The second factor was the configuration of the unbound ligand in an aqueous solution. These two factors were found to be related to the logarithm of the affinities using a simple correlation model, which could be useful when designing high affinity subtype selective bitropic ligands. While this model is based upon the interaction of arylamide phenylpiperazines with the D2 and D3 D2-like dopamine receptor subtypes, it provides insights into the complexity of the factors that define a bitropic mode of the binding at GPCRs.
中文翻译:

芳酰胺苯基哌嗪配体的类似物,用于研究影响D3多巴胺受体双向结合和受体亚型选择性的因素。
先前我们已经报道过芳基酰胺苯基哌嗪与D3和D2多巴胺受体亚型选择性结合的能力。对于这些研究,我们将LS-3-134用作原型芳基酰胺苯基哌嗪配体,因为它与D3多巴胺受体(0.17 nM)具有高亲和力结合,并且相对于D2受体具有超过150倍的结合选择性。我们的目标是研究取代的苯基哌嗪类似物在哌嗪位置的非芳香环结构的组成和大小如何影响对人D2和D3多巴胺受体的结合亲和力。已确定两个因素对于确定双亲芳基酰胺基苯基哌嗪对多巴胺D3受体亚型的结合亲和力很重要。一个因素是高度保守的残基Asp3之间盐桥的强度。32在哌嗪位置具有非芳族环的质子化氮。第二个因素是水溶液中未结合配体的构型。使用简单的相关模型,发现这两个因素与亲和力的对数有关,这在设计高亲和力亚型选择性双亲性配体时可能有用。虽然此模型基于芳基酰胺苯基哌嗪与D2和D3 D2样多巴胺受体亚型的相互作用,但它提供了对定义GPCR结合模式的双效因子的复杂性的见解。使用简单的相关模型,发现这两个因素与亲和力的对数有关,这在设计高亲和力亚型选择性双亲性配体时可能有用。虽然此模型基于芳基酰胺苯基哌嗪与D2和D3 D2样多巴胺受体亚型的相互作用,但它提供了对定义GPCR结合模式的双效因子的复杂性的见解。使用简单的相关模型,发现这两个因素与亲和力的对数相关,这在设计高亲和力亚型选择性双亲性配体时可能有用。虽然此模型基于芳基酰胺苯基哌嗪与D2和D3 D2样多巴胺受体亚型的相互作用,但它提供了对定义GPCR结合模式的双效因子的复杂性的见解。
更新日期:2018-07-16
中文翻译:

芳酰胺苯基哌嗪配体的类似物,用于研究影响D3多巴胺受体双向结合和受体亚型选择性的因素。
先前我们已经报道过芳基酰胺苯基哌嗪与D3和D2多巴胺受体亚型选择性结合的能力。对于这些研究,我们将LS-3-134用作原型芳基酰胺苯基哌嗪配体,因为它与D3多巴胺受体(0.17 nM)具有高亲和力结合,并且相对于D2受体具有超过150倍的结合选择性。我们的目标是研究取代的苯基哌嗪类似物在哌嗪位置的非芳香环结构的组成和大小如何影响对人D2和D3多巴胺受体的结合亲和力。已确定两个因素对于确定双亲芳基酰胺基苯基哌嗪对多巴胺D3受体亚型的结合亲和力很重要。一个因素是高度保守的残基Asp3之间盐桥的强度。32在哌嗪位置具有非芳族环的质子化氮。第二个因素是水溶液中未结合配体的构型。使用简单的相关模型,发现这两个因素与亲和力的对数有关,这在设计高亲和力亚型选择性双亲性配体时可能有用。虽然此模型基于芳基酰胺苯基哌嗪与D2和D3 D2样多巴胺受体亚型的相互作用,但它提供了对定义GPCR结合模式的双效因子的复杂性的见解。使用简单的相关模型,发现这两个因素与亲和力的对数有关,这在设计高亲和力亚型选择性双亲性配体时可能有用。虽然此模型基于芳基酰胺苯基哌嗪与D2和D3 D2样多巴胺受体亚型的相互作用,但它提供了对定义GPCR结合模式的双效因子的复杂性的见解。使用简单的相关模型,发现这两个因素与亲和力的对数相关,这在设计高亲和力亚型选择性双亲性配体时可能有用。虽然此模型基于芳基酰胺苯基哌嗪与D2和D3 D2样多巴胺受体亚型的相互作用,但它提供了对定义GPCR结合模式的双效因子的复杂性的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号