当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure of the CoI Intermediate of a Cobalt Pentapyridyl Catalyst for Hydrogen Evolution Revealed by Time‐Resolved X‐ray Spectroscopy
ChemSusChem ( IF 7.5 ) Pub Date : 2018-08-22 , DOI: 10.1002/cssc.201801140 Grigory Smolentsev 1, 2 , Mikhail A. Soldatov 2 , Benjamin Probst 3 , Cyril Bachmann 3 , Nicolo Azzaroli 1 , Roger Alberto 3 , Maarten Nachtegaal 1 , Jeroen A. van Bokhoven 1, 4
ChemSusChem ( IF 7.5 ) Pub Date : 2018-08-22 , DOI: 10.1002/cssc.201801140 Grigory Smolentsev 1, 2 , Mikhail A. Soldatov 2 , Benjamin Probst 3 , Cyril Bachmann 3 , Nicolo Azzaroli 1 , Roger Alberto 3 , Maarten Nachtegaal 1 , Jeroen A. van Bokhoven 1, 4
Affiliation
|
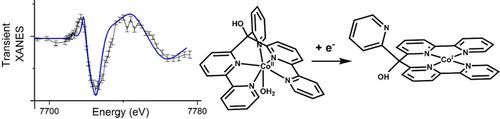
Cobalt polypyridyls are highly efficient water‐stable molecular catalysts for hydrogen evolution. The catalytic mechanism explaining their activity is under debate and the main question is the nature of the involvement of pyridyls in the proton transfer: the pentapyridyl ligand, acting as a pentadentate ligand, can provide stability to the catalyst or one of the pyridines can be involved in the proton transfer. Time‐resolved Co K‐edge X‐ray absorption spectroscopy in the microsecond time range indicates that, for the [CoII(aPPy)] catalyst (aPPy=di([2,2′‐bipyridin]‐6‐yl)(pyridin‐2‐yl)methanol), the pendant pyridine dissociates from the cobalt in the intermediate CoI state. This opens the possibility for pyridinium to act as an intramolecular proton donor. In the resting state, the catalyst returns to the original six‐coordinate high‐spin CoII state with a pentapyridyl and one water molecule coordinating to the metal center. Such a bifunctional role of the polypyridyl ligands can be exploited during further optimization of the catalyst.
中文翻译:

时间分辨X射线光谱揭示的钴五联吡啶催化氢气析出的CoI中间体的结构
聚吡啶钴是用于氢释放的高效水稳性分子催化剂。解释其活性的催化机理尚在争论中,主要问题是吡啶基参与质子转移的性质:作为五齿配体的五吡啶基配体可为催化剂提供稳定性,或可涉及一种吡啶在质子转移中。在微秒时间范围内的时间分辨Co K边缘X射线吸收光谱表明,对于[Co II(aPPy)]催化剂(aPPy = di([2,2'-联吡啶] -6-6基)(吡啶-2-基)甲醇),吡啶侧基与中间体Co I中的钴解离状态。这开辟了吡啶鎓用作分子内质子供体的可能性。在静止状态下,催化剂返回到原来的六配位高旋转Co II状态,其中戊基吡啶和一个与金属中心配位的水分子。可以在进一步优化催化剂的过程中利用聚吡啶基配体的这种双功能作用。
更新日期:2018-08-22
中文翻译:

时间分辨X射线光谱揭示的钴五联吡啶催化氢气析出的CoI中间体的结构
聚吡啶钴是用于氢释放的高效水稳性分子催化剂。解释其活性的催化机理尚在争论中,主要问题是吡啶基参与质子转移的性质:作为五齿配体的五吡啶基配体可为催化剂提供稳定性,或可涉及一种吡啶在质子转移中。在微秒时间范围内的时间分辨Co K边缘X射线吸收光谱表明,对于[Co II(aPPy)]催化剂(aPPy = di([2,2'-联吡啶] -6-6基)(吡啶-2-基)甲醇),吡啶侧基与中间体Co I中的钴解离状态。这开辟了吡啶鎓用作分子内质子供体的可能性。在静止状态下,催化剂返回到原来的六配位高旋转Co II状态,其中戊基吡啶和一个与金属中心配位的水分子。可以在进一步优化催化剂的过程中利用聚吡啶基配体的这种双功能作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号