当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design and Synthesis of DNA‐Interactive β‐Carboline–Oxindole Hybrids as Cytotoxic and Apoptosis‐Inducing Agents
ChemMedChem ( IF 3.6 ) Pub Date : 2018-08-22 , DOI: 10.1002/cmdc.201800402 Ramya Tokala 1 , Sowjanya Thatikonda 2 , Usha Sree Vanteddu 1 , Sravani Sana 1 , Chandraiah Godugu 2 , Nagula Shankaraiah 1
ChemMedChem ( IF 3.6 ) Pub Date : 2018-08-22 , DOI: 10.1002/cmdc.201800402 Ramya Tokala 1 , Sowjanya Thatikonda 2 , Usha Sree Vanteddu 1 , Sravani Sana 1 , Chandraiah Godugu 2 , Nagula Shankaraiah 1
Affiliation
|
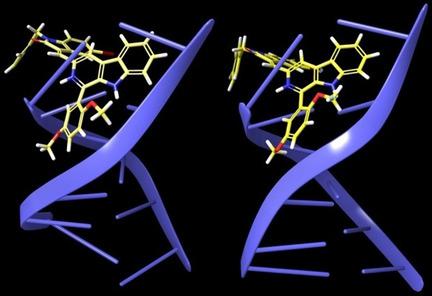
A new series of (E)‐3‐[(1‐aryl‐9H‐pyrido[3,4‐b]indol‐3‐yl)methylene]indolin‐2‐one hybrids were synthesized and evaluated for their in vitro cytotoxic activity against a panel of selected human cancer cell lines, namely, HCT‐15, HCT‐116, A549, NCI‐H460, and MCF‐7, including HFL. Among the tested compounds, (E)‐1‐benzyl‐5‐bromo‐3‐{[1‐(2,5‐dimethoxyphenyl)‐9H‐pyrido[3,4‐b]indol‐3‐yl]methylene}indolin‐2‐one (10 s) showed potent cytotoxicity against HCT‐15 cancer cells with an IC50 value of 1.43±0.26 μm and a GI50 value of 0.89±0.06 μm. Notably, induction of apoptosis by 10 s on the HCT‐15 cell line was characterized by using different staining techniques, such as acridine orange/ethidium bromide (AO/EB) and DAPI. Further, to understand the mechanism of anticancer effects, various assays such as annexin V‐FITC/PI, DCFDA, and JC‐1were performed. The flow cytometric analysis revealed that compound 10 s arrests the HCT‐15 cancer cells at the G0/G1 phase of the cell cycle. Additionally, western blot analysis indicated that treatment of 10 s on HCT‐15 cancer cells led to decreased expression of anti‐apoptotic Bcl‐2 and increased protein expression of both pro‐apoptotic Bax and caspase‐3, ‐8, and ‐9, and cleaved PARP with reference to actin. Next, a clonogenic assay revealed the inhibition of colony formation in HCT‐15 cancer cells by 10 s in a dose‐dependent manner. Moreover, upon testing on normal human lung cells (HFL), the compounds were observed to be safer with a low toxicity profile. In addition, viscosity and molecular‐docking studies showed that compound 10 s has typical intercalation with DNA.
中文翻译:

DNA相互作用的β-卡宾啉-辛醇杂合体的设计和合成作为细胞毒性和细胞凋亡诱导剂。
合成了一系列新的(E)-3-[(1-芳基-9 H-吡啶基[3,4- b ]吲哚-3-基)亚甲基]吲哚-2-酮杂种并对其体外细胞毒性进行了评估对一组选定的人类癌细胞系,即HCT-15,HCT-116,A549,NCI-H460和MCF-7(包括HFL)具有抗癌活性。在测试的化合物中,(E)-1-苄基-5-溴-3-3-[[1-(2,5-二甲氧基苯基)-9 H-吡啶基[3,4- b ]吲哚-3-基]亚甲基}二氢吲哚-2-酮(10秒)显示针对HCT-15的癌细胞的细胞毒性,其IC 50为1.43±0.26μ值米和GI 50 0.89±0.06μ值米。值得注意的是,使用不同的染色技术(如characterized啶橙/溴化乙锭(AO / EB)和DAPI)对HCT-15细胞系上10 s的凋亡诱导进行了表征。此外,为了了解抗癌作用的机制,进行了多种测定,例如膜联蛋白V-FITC / PI,DCFDA和JC-1。流式细胞仪分析表明,化合物10 s在细胞周期的G0 / G1期阻止了HCT-15癌细胞的生长。此外,蛋白质印迹分析表明处理10 sHCT-15癌细胞上的抗凋亡导致抗凋亡Bcl-2的表达降低以及促凋亡Bax和caspase-3,-8和-9的蛋白质表达增加,并且参照肌动蛋白裂解PARP。接下来,克隆形成试验揭示了HCT-15癌细胞中集落形成的抑制作用以剂量依赖性方式被抑制了10 s。此外,在正常人肺细胞(HFL)上进行测试后,发现该化合物更安全,毒性较低。此外,粘度和分子对接研究表明,化合物10 s具有与DNA的典型嵌入。
更新日期:2018-08-22
中文翻译:

DNA相互作用的β-卡宾啉-辛醇杂合体的设计和合成作为细胞毒性和细胞凋亡诱导剂。
合成了一系列新的(E)-3-[(1-芳基-9 H-吡啶基[3,4- b ]吲哚-3-基)亚甲基]吲哚-2-酮杂种并对其体外细胞毒性进行了评估对一组选定的人类癌细胞系,即HCT-15,HCT-116,A549,NCI-H460和MCF-7(包括HFL)具有抗癌活性。在测试的化合物中,(E)-1-苄基-5-溴-3-3-[[1-(2,5-二甲氧基苯基)-9 H-吡啶基[3,4- b ]吲哚-3-基]亚甲基}二氢吲哚-2-酮(10秒)显示针对HCT-15的癌细胞的细胞毒性,其IC 50为1.43±0.26μ值米和GI 50 0.89±0.06μ值米。值得注意的是,使用不同的染色技术(如characterized啶橙/溴化乙锭(AO / EB)和DAPI)对HCT-15细胞系上10 s的凋亡诱导进行了表征。此外,为了了解抗癌作用的机制,进行了多种测定,例如膜联蛋白V-FITC / PI,DCFDA和JC-1。流式细胞仪分析表明,化合物10 s在细胞周期的G0 / G1期阻止了HCT-15癌细胞的生长。此外,蛋白质印迹分析表明处理10 sHCT-15癌细胞上的抗凋亡导致抗凋亡Bcl-2的表达降低以及促凋亡Bax和caspase-3,-8和-9的蛋白质表达增加,并且参照肌动蛋白裂解PARP。接下来,克隆形成试验揭示了HCT-15癌细胞中集落形成的抑制作用以剂量依赖性方式被抑制了10 s。此外,在正常人肺细胞(HFL)上进行测试后,发现该化合物更安全,毒性较低。此外,粘度和分子对接研究表明,化合物10 s具有与DNA的典型嵌入。











































 京公网安备 11010802027423号
京公网安备 11010802027423号