当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Interface Interactions of the Bowman–Birk Inhibitor BTCI in a Ternary Complex with Trypsin and Chymotrypsin Evaluated by Semiempirical Quantum Mechanical Calculations
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2018-08-24 , DOI: 10.1002/ejoc.201800754 Diego Elias Honda 1 , João Batista Lopes Martins 2 , Manuel Mateus Ventura 1 , Saltuk Mustafa Eyrilmez 3 , Martin Lepšík 3 , Pavel Hobza 3 , Adam Pecina 3 , Sonia Maria de Freitas 1
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2018-08-24 , DOI: 10.1002/ejoc.201800754 Diego Elias Honda 1 , João Batista Lopes Martins 2 , Manuel Mateus Ventura 1 , Saltuk Mustafa Eyrilmez 3 , Martin Lepšík 3 , Pavel Hobza 3 , Adam Pecina 3 , Sonia Maria de Freitas 1
Affiliation
|
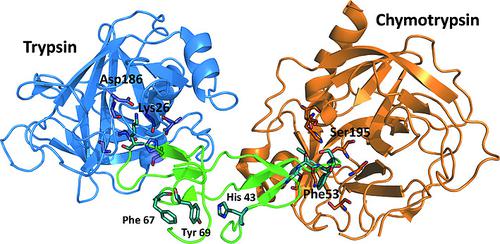
We describe the interfaces of the Bowman–Birk inhibitor (BTCI) with proteases trypsin and chymotrypsin by semiempirical quantum‐mechanical methods and give an implicit description of the water environment. The importance of amino acids at the interfaces, for example Lys26 and Phe53 in the BTCI and Asp186 (trypsin) and Ser195 (chymotrypsin), was identified and the side‐chain contributions were quantified.

中文翻译:

半经验量子力学计算评估胰蛋白酶和胰凝乳蛋白酶三元复合物中鲍曼-伯克抑制剂BTCI的界面相互作用。
我们通过半经验量子力学方法描述了鲍曼-伯克抑制剂(BTCI)与蛋白酶胰蛋白酶和胰凝乳蛋白酶的界面,并给出了水环境的隐式描述。确定了界面上氨基酸的重要性,例如BTCI和Asp186(胰蛋白酶)和Ser195(胰凝乳蛋白酶)中的Lys26和Phe53,并定量了侧链贡献。

更新日期:2018-08-24

中文翻译:

半经验量子力学计算评估胰蛋白酶和胰凝乳蛋白酶三元复合物中鲍曼-伯克抑制剂BTCI的界面相互作用。
我们通过半经验量子力学方法描述了鲍曼-伯克抑制剂(BTCI)与蛋白酶胰蛋白酶和胰凝乳蛋白酶的界面,并给出了水环境的隐式描述。确定了界面上氨基酸的重要性,例如BTCI和Asp186(胰蛋白酶)和Ser195(胰凝乳蛋白酶)中的Lys26和Phe53,并定量了侧链贡献。




























 京公网安备 11010802027423号
京公网安备 11010802027423号