当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Palladium-Catalyzed Ullmann Cross-Coupling Reaction: A Modern Variant on a Time-Honored Process
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-07-16 00:00:00 , DOI: 10.1021/acs.accounts.8b00169 Faiyaz Khan 1 , Michael Dlugosch 1 , Xin Liu 1 , Martin G. Banwell 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-07-16 00:00:00 , DOI: 10.1021/acs.accounts.8b00169 Faiyaz Khan 1 , Michael Dlugosch 1 , Xin Liu 1 , Martin G. Banwell 1
Affiliation
|
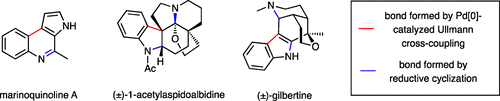
Cross-coupling reactions, especially those that are catalyzed by palladium, have revolutionized the way in which carbon–carbon bonds can be formed. The most commonly deployed variants of such processes are the Suzuki–Miyaura, Mizoroki–Heck, Stille, and Negishi cross-coupling reactions, and these normally involve the linking of an organohalide or pseudohalide (such as a triflate or nonaflate) with an organo-metallic or -metalloid such as an organo-boron, -magnesium, -tin, or -zinc species. Since the latter type of coupling partner is often prepared from the corresponding halide, methods that allow for the direct cross-coupling of two distinct halogen-containing compounds would provide valuable and more atom-economical capacities for the formation of carbon–carbon bonds. While the venerable Ullmann reaction can in principle achieve this, it has a number of drawbacks, the most significant of which is that homocoupling of the reaction partners is a competitive, if not the dominant, process. Furthermore, such reactions normally occur only under forcing conditions (viz., often at temperatures in excess of 250 °C). As such, the Ullmann reaction has seen only limited application in this regard, especially as a mid- to late-stage feature of complex natural product synthesis. This Account details the development of the palladium-catalyzed Ullmann cross-coupling reaction as a useful method for the assembly of a range of heterocyclic systems relevant to medicinal and/or natural products chemistry. These couplings normally proceed under relatively mild conditions (<100 °C) over short periods of time and, usually, to the exclusion of (unwanted) homocoupling events. The keys to success are the appropriate choice of coupling partners, the form of the copper metal employed, and the choice of reaction solvent.
中文翻译:

钯催化的Ullmann交叉偶联反应:基于时间的过程的现代变体
交叉偶联反应,特别是钯催化的交叉偶联反应,彻底改变了碳-碳键的形成方式。这种工艺最常用的变体是Suzuki–Miyaura,Mizuroki–Heck,Stille和Negishi交叉偶联反应,通常涉及有机卤化物或假卤化物(例如三氟甲磺酸盐或九氟乙磺酸盐)与有机卤化物的连接。金属或-类金属,例如有机硼,-镁,-锡或-锌。由于后一种偶合剂通常是由相应的卤化物制备的,因此允许两种截然不同的含卤素化合物直接交叉偶合的方法将为形成碳-碳键提供有价值且更具原子经济性的能力。尽管古老的Ullmann反应原则上可以实现这一目标,它具有许多缺点,其中最显着的是,反应伙伴的均偶联是竞争性的过程,即使不是主导性过程。此外,这种反应通常仅在强迫条件下发生(即,通常在超过250°C的温度下)。因此,在这方面,Ullmann反应仅得到了有限的应用,特别是作为复杂的天然产物合成的中后期特征。该帐户详细介绍了钯催化的Ullmann交叉偶联反应的发展,该反应是组装与医学和/或天然产物化学相关的一系列杂环系统的有用方法。这些耦合通常在相对温和的条件下(<100°C)在短时间内进行,通常会排除(不需要的)同质耦合事件。
更新日期:2018-07-16
中文翻译:

钯催化的Ullmann交叉偶联反应:基于时间的过程的现代变体
交叉偶联反应,特别是钯催化的交叉偶联反应,彻底改变了碳-碳键的形成方式。这种工艺最常用的变体是Suzuki–Miyaura,Mizuroki–Heck,Stille和Negishi交叉偶联反应,通常涉及有机卤化物或假卤化物(例如三氟甲磺酸盐或九氟乙磺酸盐)与有机卤化物的连接。金属或-类金属,例如有机硼,-镁,-锡或-锌。由于后一种偶合剂通常是由相应的卤化物制备的,因此允许两种截然不同的含卤素化合物直接交叉偶合的方法将为形成碳-碳键提供有价值且更具原子经济性的能力。尽管古老的Ullmann反应原则上可以实现这一目标,它具有许多缺点,其中最显着的是,反应伙伴的均偶联是竞争性的过程,即使不是主导性过程。此外,这种反应通常仅在强迫条件下发生(即,通常在超过250°C的温度下)。因此,在这方面,Ullmann反应仅得到了有限的应用,特别是作为复杂的天然产物合成的中后期特征。该帐户详细介绍了钯催化的Ullmann交叉偶联反应的发展,该反应是组装与医学和/或天然产物化学相关的一系列杂环系统的有用方法。这些耦合通常在相对温和的条件下(<100°C)在短时间内进行,通常会排除(不需要的)同质耦合事件。











































 京公网安备 11010802027423号
京公网安备 11010802027423号