当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploiting the Thiobarbituric Acid Scaffold for Antibacterial Activity
ChemMedChem ( IF 3.6 ) Pub Date : 2018-08-13 , DOI: 10.1002/cmdc.201800414 Anamika Sharma 1 , Sikabwe Noki 1 , Sizwe J. Zamisa 2 , Heba A. Hazzah 1, 3 , Zainab M. Almarhoon 4 , Ayman El-Faham 4, 5 , Beatriz G. de la Torre 6 , Fernando Albericio 2, 4, 7, 8
ChemMedChem ( IF 3.6 ) Pub Date : 2018-08-13 , DOI: 10.1002/cmdc.201800414 Anamika Sharma 1 , Sikabwe Noki 1 , Sizwe J. Zamisa 2 , Heba A. Hazzah 1, 3 , Zainab M. Almarhoon 4 , Ayman El-Faham 4, 5 , Beatriz G. de la Torre 6 , Fernando Albericio 2, 4, 7, 8
Affiliation
|
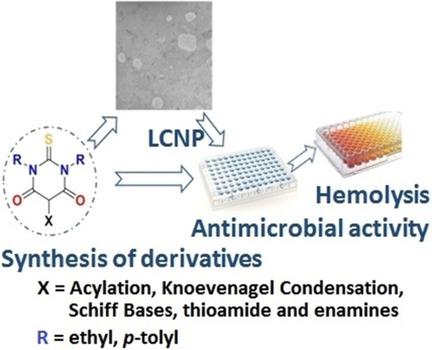
Thiobarbituric acid (TBA) has been considered a privileged structure for developing antimicrobial agents. Diversity was obtained at positions N and at C5 through acylation, Schiff base formation, Knoevenagel condensation, and thioamide and enamine formation. The present work describes the synthesis of small libraries based on the TBA moiety and above‐mentioned reactions. Preliminary antimicrobial activity screening of the prepared compounds against selected bacteria (both Gram‐positive and ‐negative) showed the best results for the Boc‐Phe‐TBA derivative. These results could be useful for designing and building libraries based on other amino acids with distinct protecting groups.
中文翻译:

利用硫代巴比妥酸支架进行抗菌活性
硫代巴比妥酸(TBA)被认为是开发抗菌剂的特权结构。通过酰化,席夫碱形成,Knoevenagel缩合以及硫酰胺和烯胺形成,在位置N和C5处获得了多样性。本工作描述了基于TBA部分和上述反应的小文库的合成。初步制备的化合物对所选细菌(革兰氏阳性和阴性)的抗菌活性筛选显示,Boc-Phe-TBA衍生物的最佳结果。这些结果对于基于具有不同保护基团的其他氨基酸的设计和构建文库可能是有用的。
更新日期:2018-08-13
中文翻译:

利用硫代巴比妥酸支架进行抗菌活性
硫代巴比妥酸(TBA)被认为是开发抗菌剂的特权结构。通过酰化,席夫碱形成,Knoevenagel缩合以及硫酰胺和烯胺形成,在位置N和C5处获得了多样性。本工作描述了基于TBA部分和上述反应的小文库的合成。初步制备的化合物对所选细菌(革兰氏阳性和阴性)的抗菌活性筛选显示,Boc-Phe-TBA衍生物的最佳结果。这些结果对于基于具有不同保护基团的其他氨基酸的设计和构建文库可能是有用的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号