当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Decoration of an α-Resorcylate Nucleus as Part of the Manufacture of a Glucokinase Activator
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-07-13 00:00:00 , DOI: 10.1021/acs.oprd.8b00175 Phillip Hopes 1 , Thomas Langer 2 , Kirsty Millard 2 , Alan Steven 2
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-07-13 00:00:00 , DOI: 10.1021/acs.oprd.8b00175 Phillip Hopes 1 , Thomas Langer 2 , Kirsty Millard 2 , Alan Steven 2
Affiliation
|
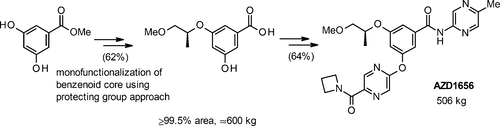
The need to define a set of processes for the manufacture of a glucokinase activator called for an evaluation of different strategies to differentiate the hydroxyls of an α-resorcylic acid derivative. While direct functionalization proved possible, it did not allow access to crystalline intermediates that offered control over the rejection of process impurities. The strategy taken forward involved the installation of a benzoyl protecting group using careful control of pH in order to achieve useful levels of selectivity. This allowed the remaining α-resorcylate hydroxyl to be functionalized using a Mitsunobu reaction, with liquid–liquid partitioning being used to separate downstream intermediates of interest away from the redox byproducts of this reaction. Downstream challenges that were overcome in order to deliver a commercially viable means of manufacturing the API included developing an amidation reaction with a poorly reactive aminopyrazine coupling partner.
中文翻译:

作为葡萄糖激酶激活剂生产的一部分,对α-间苯二酚核的修饰
需要定义一组用于制造葡萄糖激酶激活剂的方法,要求评估不同的策略以区分α-间苯二酸衍生物的羟基。尽管直接功能化被证明是可行的,但它不允许进入提供控制工艺杂质排除的结晶中间体。所采取的策略涉及通过小心控制pH值来安装苯甲酰基保护基,以达到有用的选择性水平。这允许使用Mitsunobu反应将剩余的α-间苯二酚羟基官能化,并使用液-液分配法将感兴趣的下游中间体与该反应的氧化还原副产物分离。
更新日期:2018-07-13
中文翻译:

作为葡萄糖激酶激活剂生产的一部分,对α-间苯二酚核的修饰
需要定义一组用于制造葡萄糖激酶激活剂的方法,要求评估不同的策略以区分α-间苯二酸衍生物的羟基。尽管直接功能化被证明是可行的,但它不允许进入提供控制工艺杂质排除的结晶中间体。所采取的策略涉及通过小心控制pH值来安装苯甲酰基保护基,以达到有用的选择性水平。这允许使用Mitsunobu反应将剩余的α-间苯二酚羟基官能化,并使用液-液分配法将感兴趣的下游中间体与该反应的氧化还原副产物分离。











































 京公网安备 11010802027423号
京公网安备 11010802027423号