当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Small molecule cores demonstrate non-competitive inhibition of lactate dehydrogenase†
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2018-07-13 00:00:00 , DOI: 10.1039/c8md00309b Brooke A. Andrews 1, 2, 3, 4 , R. Brian Dyer 1, 2, 3, 4
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2018-07-13 00:00:00 , DOI: 10.1039/c8md00309b Brooke A. Andrews 1, 2, 3, 4 , R. Brian Dyer 1, 2, 3, 4
Affiliation
|
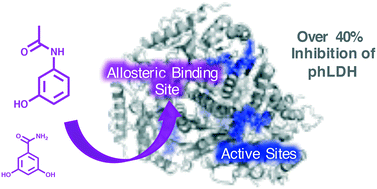
Lactate dehydrogenase (LDH) has recently garnered attention as an attractive target for cancer therapies, owing to the enzyme's critical role in cellular metabolism. Current inhibition strategies, employing substrate or cofactor analogues, are insufficiently specific for use as pharmaceutical agents. The possibility of allosteric inhibition of LDH was postulated on the basis of theoretical docking studies of a small molecule inhibitor to LDH. The present study examined structural analogues of this proposed inhibitor to gauge its potency and attempt to elucidate the molecular mechanism of action. These analogues display encouraging in vitro inhibition of porcine heart LDH, including micromolar Ki values and a maximum inhibition of up to 50% in the steady state. Furthermore, Michaelis–Menten kinetics and fluorescence data both suggest the simple, acetaminophen derivatives are non-competitive in binding to the enzyme. Kinetic comparisons of a panel of increasingly decorated structural analogues imply that the binding is specific, and the small molecule core provides a privileged scaffold for further pharmaceutical development of a novel, allosteric drug.
中文翻译:

小分子核心显示出非竞争性抑制乳酸脱氢酶的作用†
乳酸脱氢酶(LDH)由于其在细胞代谢中的关键作用,最近已引起人们的关注,成为一种有吸引力的癌症治疗靶标。使用底物或辅因子类似物的当前抑制策略对于用作药物而言不够特异性。根据对LDH的小分子抑制剂的理论对接研究,推测了对LDH的变构抑制作用的可能性。本研究检查了该拟议抑制剂的结构类似物,以评估其效力并试图阐明其作用的分子机制。这些类似物表现出令人鼓舞的体外抑制猪心脏LDH的能力,包括微摩尔K i值和稳态下的最大抑制高达50%。此外,Michaelis-Menten动力学和荧光数据均表明,简单的对乙酰氨基酚衍生物与酶的结合不具有竞争性。一组装饰越来越多的结构类似物的动力学比较表明该结合是特异性的,并且小分子核心为新的变构药物的进一步药物开发提供了一种有利的支架。
更新日期:2018-07-13
中文翻译:

小分子核心显示出非竞争性抑制乳酸脱氢酶的作用†
乳酸脱氢酶(LDH)由于其在细胞代谢中的关键作用,最近已引起人们的关注,成为一种有吸引力的癌症治疗靶标。使用底物或辅因子类似物的当前抑制策略对于用作药物而言不够特异性。根据对LDH的小分子抑制剂的理论对接研究,推测了对LDH的变构抑制作用的可能性。本研究检查了该拟议抑制剂的结构类似物,以评估其效力并试图阐明其作用的分子机制。这些类似物表现出令人鼓舞的体外抑制猪心脏LDH的能力,包括微摩尔K i值和稳态下的最大抑制高达50%。此外,Michaelis-Menten动力学和荧光数据均表明,简单的对乙酰氨基酚衍生物与酶的结合不具有竞争性。一组装饰越来越多的结构类似物的动力学比较表明该结合是特异性的,并且小分子核心为新的变构药物的进一步药物开发提供了一种有利的支架。











































 京公网安备 11010802027423号
京公网安备 11010802027423号