当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric iodine catalysis-mediated enantioselective oxidative transformations
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-07-13 00:00:00 , DOI: 10.1039/c8ob01378k Aurélie Claraz 1, 2, 3, 4, 5 , Géraldine Masson 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-07-13 00:00:00 , DOI: 10.1039/c8ob01378k Aurélie Claraz 1, 2, 3, 4, 5 , Géraldine Masson 1, 2, 3, 4, 5
Affiliation
|
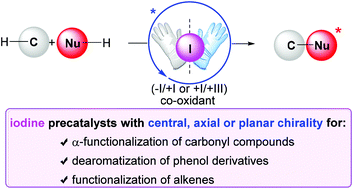
The implementation of chiral iodine catalysis has tremendously been developed in the field of asymmetric synthesis over the past decade. It enables the stereoselective creation of C–O as well as C–C, C–N and C–X (X = halogen) bonds through oxidative transformations. Thanks to the low toxicity and ease of handling of iodine compounds, this strategy offers many advantages over classical metal-catalyzed oxidations with chiral ligands. The approaches rely on iodine(I/III) or (−I/+I) catalysis by using a chiral aryliodine or ammonium iodide respectively in combination with a suitable terminal oxidant. As such, the design of iodine compounds with central, axial or even planar chirality has allowed us to achieve high enantioselectivities. The goal of this review is to cover the different chiral iodine compound-catalyzed oxidative transformations including α-functionalization of carbonyl compounds, dearomatization of phenol derivatives and difunctionalization of alkenes which should demonstrate that iodine catalysis has now found its place in the realm of asymmetric organocatalysis.
中文翻译:

不对称碘催化介导的对映选择性氧化转化
在过去的十年中,在不对称合成领域中已经极大地发展了手性碘催化的实现。它可以通过氧化转化立体选择性地生成C–O以及C–C,C–N和C–X(X =卤素)键。由于碘化合物的低毒性和易处理性,与采用手性配体的经典金属催化氧化方法相比,该策略具有许多优势。这些方法依靠碘(I / III)或(− I / + I)分别使用手性芳碘或碘化铵与合适的终端氧化剂结合进行催化。因此,具有中心,轴向或什至平面手性的碘化合物的设计使我们能够实现高对映选择性。这篇综述的目的是涵盖不同的手性碘化合物催化的氧化转化,包括羰基化合物的α-官能化,苯酚衍生物的脱芳烃化和烯烃的双官能化,这应证明碘催化已在不对称有机催化领域中找到了自己的位置。 。
更新日期:2018-07-13
中文翻译:

不对称碘催化介导的对映选择性氧化转化
在过去的十年中,在不对称合成领域中已经极大地发展了手性碘催化的实现。它可以通过氧化转化立体选择性地生成C–O以及C–C,C–N和C–X(X =卤素)键。由于碘化合物的低毒性和易处理性,与采用手性配体的经典金属催化氧化方法相比,该策略具有许多优势。这些方法依靠碘(I / III)或(− I / + I)分别使用手性芳碘或碘化铵与合适的终端氧化剂结合进行催化。因此,具有中心,轴向或什至平面手性的碘化合物的设计使我们能够实现高对映选择性。这篇综述的目的是涵盖不同的手性碘化合物催化的氧化转化,包括羰基化合物的α-官能化,苯酚衍生物的脱芳烃化和烯烃的双官能化,这应证明碘催化已在不对称有机催化领域中找到了自己的位置。 。









































 京公网安备 11010802027423号
京公网安备 11010802027423号