当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nα-Amino acid containing privileged structures: design, synthesis and use in solid-phase peptide synthesis†
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-07-12 00:00:00 , DOI: 10.1039/c8ob01485j Eva Schütznerová 1, 2, 3, 4, 5 , Adam Přibylka 3, 5, 6, 7, 8 , Viktor Krchňák 3, 5, 6, 7, 8
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-07-12 00:00:00 , DOI: 10.1039/c8ob01485j Eva Schütznerová 1, 2, 3, 4, 5 , Adam Přibylka 3, 5, 6, 7, 8 , Viktor Krchňák 3, 5, 6, 7, 8
Affiliation
|
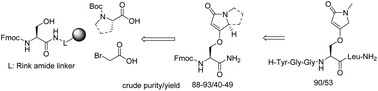
Fmoc-protected Nα-amino acid containing heterocyclic privileged structures, O-(1-methyl-5-oxo-2,5-dihydro-1H-pyrrol-3-yl)-L-serine and O-((S)-5-oxo-2,3,5,7a-tetrahydro-1H-pyrrolizin-7-yl)-L-serine, were synthesized on the solid phase from simple commercially available building blocks under mild conditions. The amino acid side-chain is composed of tetramic acid, a natural product derived privileged structure. The key transformation was the formation of cyclic enol ethers via nonclassical Wittig olefinations of the esters. Solid-phase synthesis represents a method of choice, particularly for the synthesis of peptides. This route is compatible with traditional Merrifield solid-phase peptide synthesis (SPPS), as documented on the preparation of the pentapeptide Leu-enkephalin amide H-Tyr-Gly-Gly-Phe-Leu-NH2 with Phe or Tyr replaced by a novel amino acid.
中文翻译:

Ñ α含特权结构氨基酸:设计,合成和使用固相肽合成†
Fmoc-保护的Ñ α含氮杂环特权结构,α-氨基酸ø - (1-甲基-5-氧代-2,5-二氢- 1 H ^ -吡咯-3-基) -大号-丝氨酸和ø - ((小号)在温和条件下,由简单的市售结构单元在固相上合成了-5-oxo-2,3,5,7a-四氢-1 H-吡咯嗪-7-基)-L-丝氨酸。氨基酸侧链由天然产物衍生的特权结构四酸组成。关键的转变是通过酯的非经典维蒂希烯化反应。固相合成代表一种选择的方法,特别是对于肽的合成。如制备五肽亮氨酸-脑啡肽酰胺H-Tyr-Gly-Gly-Phe-Leu-NH 2并用新型Phe或Tyr取代五肽所记录的,该路线与传统的Merrifield固相肽合成(SPPS)兼容氨基酸。
更新日期:2018-07-12
中文翻译:

Ñ α含特权结构氨基酸:设计,合成和使用固相肽合成†
Fmoc-保护的Ñ α含氮杂环特权结构,α-氨基酸ø - (1-甲基-5-氧代-2,5-二氢- 1 H ^ -吡咯-3-基) -大号-丝氨酸和ø - ((小号)在温和条件下,由简单的市售结构单元在固相上合成了-5-oxo-2,3,5,7a-四氢-1 H-吡咯嗪-7-基)-L-丝氨酸。氨基酸侧链由天然产物衍生的特权结构四酸组成。关键的转变是通过酯的非经典维蒂希烯化反应。固相合成代表一种选择的方法,特别是对于肽的合成。如制备五肽亮氨酸-脑啡肽酰胺H-Tyr-Gly-Gly-Phe-Leu-NH 2并用新型Phe或Tyr取代五肽所记录的,该路线与传统的Merrifield固相肽合成(SPPS)兼容氨基酸。











































 京公网安备 11010802027423号
京公网安备 11010802027423号