当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrogenation of CO2 into hydrocarbons: enhanced catalytic activity over Fe-based Fischer–Tropsch catalysts†
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-07-13 00:00:00 , DOI: 10.1039/c8cy00850g Feng Jiang 1, 2, 3, 4, 5 , Bing Liu 1, 2, 3, 4, 5 , Shunshun Geng 1, 2, 3, 4, 5 , Yuebing Xu 1, 2, 3, 4, 5 , Xiaohao Liu 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-07-13 00:00:00 , DOI: 10.1039/c8cy00850g Feng Jiang 1, 2, 3, 4, 5 , Bing Liu 1, 2, 3, 4, 5 , Shunshun Geng 1, 2, 3, 4, 5 , Yuebing Xu 1, 2, 3, 4, 5 , Xiaohao Liu 1, 2, 3, 4, 5
Affiliation
|
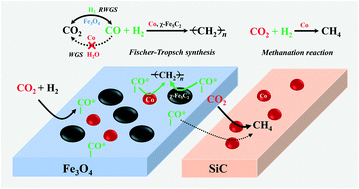
CO2-FTS is one of the most practical routes for converting CO2 into valuable chemicals and fuels to reduce CO2 emissions. However, efficient conversion remains a challenge because of its chemically inert property. Previously, we have reported a remarkably enhanced CO2 conversion efficiency by driving the RWGS reaction via the removal of large amounts of formed water in the reaction system. In this study, we propose to effectively enhance the CO2 conversion by promoting the conversion of the CO intermediate in the FTS stage. For this purpose, a K or/and Co (Ru) component is introduced onto the precipitated Fe catalysts. Results show that the addition of K obviously increases the CO2 conversion due to the promoted formation of iron carbide sites as the active FTS reaction phase. Moreover, the selectivity to C2+ hydrocarbons, especially lower olefins, can be substantially enhanced owing to the electron donation from K to Fe which promotes chain growth and suppresses the direct hydrogenation of Fe-(CH2)n intermediates. The addition of Co (Ru) that has no WGS activity could further remarkably enhance the CO2 conversion for Co (Ru) only promotes the FTS reaction rate of the CO intermediate without catalyzing the conversion of CO into CO2. Furthermore, the effects of the intimacy between Co and Fe sites from the nanoscale to the meter scale have also been investigated. The results display that the intimate contact between Fe and Co sites favors a higher selectivity to C2+ hydrocarbons, which is ascribed to the easy spillover of the CO intermediate from Fe3O4 to Co sites and thereby yielding a higher CO concentration over Co sites. In contrast, the increase in the distance between Fe and Co sites leads to a remarkably higher selectivity to CH4, which could be because the direct methanation of CO2 is enhanced and the chain growth possibility of the FTS reaction is reduced due to the lower CO concentration over Co sites.
中文翻译:

将CO 2加氢成碳氢化合物:比铁基费-托催化剂更强的催化活性†
CO 2 -FTS是将CO 2转化为有价值的化学物质和燃料以减少CO 2排放的最实用途径之一。然而,由于其化学惰性,有效的转化仍然是一个挑战。以前,我们已经报道了通过去除反应体系中大量的生成水来驱动RWGS反应,从而显着提高了CO 2转化效率。在这项研究中,我们建议通过在FTS阶段促进CO中间体的转化来有效地提高CO 2转化率。为此,将K或/和Co(Ru)组分引入到沉淀的Fe催化剂上。结果表明,添加钾明显增加了CO由于促进了作为活性FTS反应相的碳化铁位点的形成,因此发生了2转化。此外,由于从K到Fe的电子给电子,这促进了链增长并抑制了Fe-(CH 2) n中间体的直接氢化,因此可以大大提高对C 2+烃,特别是低级烯烃的选择性。添加不具有WGS活性的Co(Ru)可以进一步显着提高Co(Ru)的CO 2转化率,仅提高CO中间体的FTS反应速率,而不会催化CO转化为CO 2。此外,还研究了从纳米级到米级的Co和Fe位点之间亲密性的影响。结果表明,Fe和Co位点之间的紧密接触有利于对C 2+烃的更高选择性,这归因于CO中间体易于从Fe 3 O 4溢出到Co位点,从而产生比Co高的CO浓度。网站。相反,Fe和Co位点之间距离的增加导致对CH 4的选择性显着提高,这可能是因为CO 2的直接甲烷化作用增强,并且由于较低的FTS反应链增长的可能性而降低了。 Co位点上的CO浓度。
更新日期:2018-07-13
中文翻译:

将CO 2加氢成碳氢化合物:比铁基费-托催化剂更强的催化活性†
CO 2 -FTS是将CO 2转化为有价值的化学物质和燃料以减少CO 2排放的最实用途径之一。然而,由于其化学惰性,有效的转化仍然是一个挑战。以前,我们已经报道了通过去除反应体系中大量的生成水来驱动RWGS反应,从而显着提高了CO 2转化效率。在这项研究中,我们建议通过在FTS阶段促进CO中间体的转化来有效地提高CO 2转化率。为此,将K或/和Co(Ru)组分引入到沉淀的Fe催化剂上。结果表明,添加钾明显增加了CO由于促进了作为活性FTS反应相的碳化铁位点的形成,因此发生了2转化。此外,由于从K到Fe的电子给电子,这促进了链增长并抑制了Fe-(CH 2) n中间体的直接氢化,因此可以大大提高对C 2+烃,特别是低级烯烃的选择性。添加不具有WGS活性的Co(Ru)可以进一步显着提高Co(Ru)的CO 2转化率,仅提高CO中间体的FTS反应速率,而不会催化CO转化为CO 2。此外,还研究了从纳米级到米级的Co和Fe位点之间亲密性的影响。结果表明,Fe和Co位点之间的紧密接触有利于对C 2+烃的更高选择性,这归因于CO中间体易于从Fe 3 O 4溢出到Co位点,从而产生比Co高的CO浓度。网站。相反,Fe和Co位点之间距离的增加导致对CH 4的选择性显着提高,这可能是因为CO 2的直接甲烷化作用增强,并且由于较低的FTS反应链增长的可能性而降低了。 Co位点上的CO浓度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号