当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High-Efficiency Electrosynthesis of Ammonia with High Selectivity under Ambient Conditions Enabled by VN Nanosheet Array
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2018-07-13 00:00:00 , DOI: 10.1021/acssuschemeng.8b01261 Rong Zhang 1, 2 , Ya Zhang 1, 2 , Xiang Ren 1 , Guanwei Cui 3 , Abdullah M. Asiri 4 , Baozhan Zheng 2 , Xuping Sun 1, 2
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2018-07-13 00:00:00 , DOI: 10.1021/acssuschemeng.8b01261 Rong Zhang 1, 2 , Ya Zhang 1, 2 , Xiang Ren 1 , Guanwei Cui 3 , Abdullah M. Asiri 4 , Baozhan Zheng 2 , Xuping Sun 1, 2
Affiliation
|
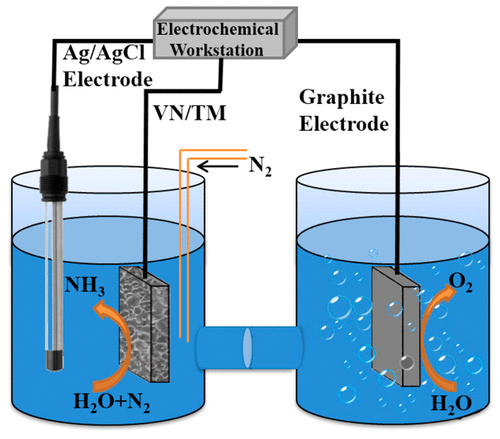
The development of efficient earth-abundant electrocatalysts for N2 reduction to ammonia (NH3) under ambient conditions is critical for achieving a low-carbon and sustainable-energy society. Herein, we report the development of VN nanosheet array on Ti mesh as an active and selective electrocatalyst for N2 reduction reaction (NRR) in acid at room temperature and atmospheric pressure in 0.1 M HCl. A rate of NH3 formation of 8.40 × 10–11 mol s–1 cm–2 is obtained at −0.50 V with a Faradaic efficiency of 2.25%. Notably, such catalyst material also exhibits high selectivity (no formation of N2H4) and electrochemical stability. Theoretical and experiment results suggest that VN catalyzes NRR via a Mars–van Krevelen mechanism. This study would offers the potential of utilization of attractive 3D catalyst electrode toward efficient NH3 synthesis for applications.
中文翻译:

VN纳米片阵列在环境条件下高效选择性高效合成氨
对于在环境条件下将N 2还原为氨(NH 3)的高效富含地球的电催化剂的开发对于实现低碳和可持续能源社会至关重要。在本文中,我们报道了在Ti网上VN纳米片阵列的发展,该活性是在室温和大气压下于0.1 M HCl中的N 2还原反应(NRR)在酸中的活性和选择性电催化剂。在-0.50 V时,法拉第效率为2.25%,可形成8.40×10 –11 mol s –1 cm –2的NH 3。明显地,这种催化剂材料还表现出高选择性(不形成N 2 H 4)。)和电化学稳定性。理论和实验结果表明,VN通过Mars-van Krevelen机制催化NRR。这项研究将提供诱人的3D催化剂电极在应用中实现高效NH 3合成的潜力。
更新日期:2018-07-13
中文翻译:

VN纳米片阵列在环境条件下高效选择性高效合成氨
对于在环境条件下将N 2还原为氨(NH 3)的高效富含地球的电催化剂的开发对于实现低碳和可持续能源社会至关重要。在本文中,我们报道了在Ti网上VN纳米片阵列的发展,该活性是在室温和大气压下于0.1 M HCl中的N 2还原反应(NRR)在酸中的活性和选择性电催化剂。在-0.50 V时,法拉第效率为2.25%,可形成8.40×10 –11 mol s –1 cm –2的NH 3。明显地,这种催化剂材料还表现出高选择性(不形成N 2 H 4)。)和电化学稳定性。理论和实验结果表明,VN通过Mars-van Krevelen机制催化NRR。这项研究将提供诱人的3D催化剂电极在应用中实现高效NH 3合成的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号