当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Modular Brush Polymer–Protein Hybrids Using Diazotransfer and Copper Click Chemistry
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-07-12 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00309 Luis A. Navarro 1 , Daniel L. French 1 , Stefan Zauscher 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-07-12 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00309 Luis A. Navarro 1 , Daniel L. French 1 , Stefan Zauscher 1
Affiliation
|
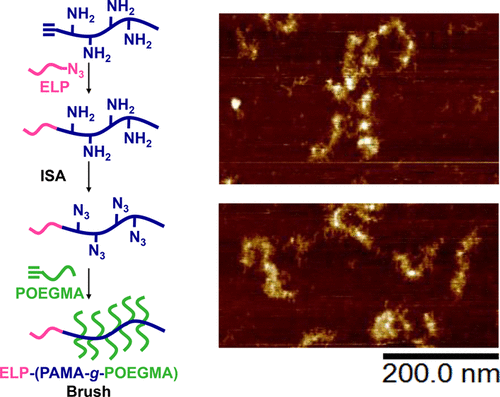
Proteoglycans are important brush-like biomacromolecules, which serve a variety of functions in the human body. While protein–bottlebrush hybrids are promising proteoglycan mimics, many challenges still exist to robustly produce such polymers. In this paper, we report the modular synthesis of protein–brush hybrids containing elastin-like polypeptides (ELP) as model proteins by copper-catalyzed azide–alkyne cycloaddition. We exploit the recently discovered imidazole-1-sulfonyl azide (ISA) in a diazotransfer reaction to introduce an N-terminal azide onto an ELP. Next, we use a click reaction to couple the azido-ELP to an alkyne-terminated amine-rich polymer followed by a second diazotransfer step to produce an azide-rich backbone that serves as a scaffold. Finally, we used a second click reaction to graft alkyne-terminated poly(oligoethylene glycol methacrylate) (POEGMA) bristles to the azide-rich backbone to produce the final protein–bottlebrush hybrid. We demonstrate the effectiveness of this synthetic path at each step through careful characterization with 1H NMR, FTIR, GPC, and diagnostic test reactions on SDS-PAGE. Final reaction products could be consistently obtained for a variety of different molecular weight backbones with final total grafting efficiencies around 70%. The high-yielding reactions employed in this highly modular approach allow for the synthesis of protein–bottlebrush hybrids with different proteins and brush polymers. Additionally, the mild reaction conditions used have the potential to avoid damage to proteins during synthesis.
中文翻译:

用重氮转移和铜点击化学合成模块化刷式聚合物-蛋白质杂化物
蛋白聚糖是重要的刷状生物大分子,在人体中具有多种功能。尽管蛋白-瓶杂种是有前途的蛋白聚糖模拟物,但要可靠地生产这种聚合物,仍然存在许多挑战。在本文中,我们报告了通过铜催化的叠氮化物-炔烃环加成反应,将包含弹性蛋白样多肽(ELP)作为模型蛋白的蛋白质-刷子杂种进行模块化合成。我们在重氮转移反应中利用最近发现的咪唑-1-磺酰叠氮化物(ISA)将N末端叠氮化物引入到ELP上。接下来,我们使用点击反应将叠氮基ELP偶联至炔烃封端的富含胺的聚合物,然后进行第二次重氮转移步骤,以生产充当骨架的富含叠氮化物的骨架。最后,我们使用第二次点击反应将炔烃封端的聚(甲基丙烯酸低聚乙二醇酯)(POEGMA)刷毛嫁接到富含叠氮化物的骨架上,从而生产出最终的蛋白质-牙刷混合物。我们通过仔细的表征,证明了这一合成路径在每个步骤中的有效性。1 H NMR,FTIR,GPC和SDS-PAGE上的诊断测试反应。对于各种不同分子量的主链,可以始终获得最终反应产物,最终总接枝效率约为70%。在这种高度模块化的方法中使用的高产量反应可以合成具有不同蛋白质和刷子聚合物的蛋白质-刷子杂种。另外,所使用的温和的反应条件具有避免在合成过程中损坏蛋白质的潜力。
更新日期:2018-07-12
中文翻译:

用重氮转移和铜点击化学合成模块化刷式聚合物-蛋白质杂化物
蛋白聚糖是重要的刷状生物大分子,在人体中具有多种功能。尽管蛋白-瓶杂种是有前途的蛋白聚糖模拟物,但要可靠地生产这种聚合物,仍然存在许多挑战。在本文中,我们报告了通过铜催化的叠氮化物-炔烃环加成反应,将包含弹性蛋白样多肽(ELP)作为模型蛋白的蛋白质-刷子杂种进行模块化合成。我们在重氮转移反应中利用最近发现的咪唑-1-磺酰叠氮化物(ISA)将N末端叠氮化物引入到ELP上。接下来,我们使用点击反应将叠氮基ELP偶联至炔烃封端的富含胺的聚合物,然后进行第二次重氮转移步骤,以生产充当骨架的富含叠氮化物的骨架。最后,我们使用第二次点击反应将炔烃封端的聚(甲基丙烯酸低聚乙二醇酯)(POEGMA)刷毛嫁接到富含叠氮化物的骨架上,从而生产出最终的蛋白质-牙刷混合物。我们通过仔细的表征,证明了这一合成路径在每个步骤中的有效性。1 H NMR,FTIR,GPC和SDS-PAGE上的诊断测试反应。对于各种不同分子量的主链,可以始终获得最终反应产物,最终总接枝效率约为70%。在这种高度模块化的方法中使用的高产量反应可以合成具有不同蛋白质和刷子聚合物的蛋白质-刷子杂种。另外,所使用的温和的反应条件具有避免在合成过程中损坏蛋白质的潜力。










































 京公网安备 11010802027423号
京公网安备 11010802027423号