当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Open and Closed Form of Maltose Binding Protein in Its Native and Molten Globule State As Studied by Electron Paramagnetic Resonance Spectroscopy
Biochemistry ( IF 2.9 ) Pub Date : 2018-07-13 00:00:00 , DOI: 10.1021/acs.biochem.8b00322 Benjamin Selmke 1 , Peter P Borbat 2 , Chen Nickolaus 1 , Raghavan Varadarajan 3 , Jack H Freed 2 , Wolfgang E Trommer 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-07-13 00:00:00 , DOI: 10.1021/acs.biochem.8b00322 Benjamin Selmke 1 , Peter P Borbat 2 , Chen Nickolaus 1 , Raghavan Varadarajan 3 , Jack H Freed 2 , Wolfgang E Trommer 1
Affiliation
|
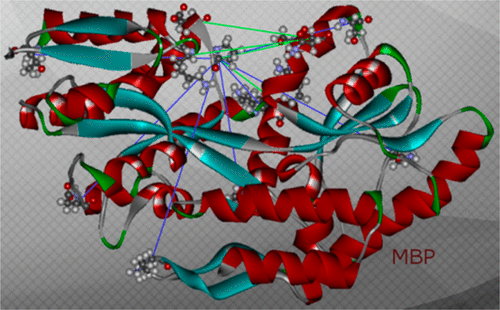
An intensively investigated intermediate state of protein folding is the molten globule (MG) state, which contains secondary but hardly any tertiary structure. In previous work, we have determined the distances between interacting spins within maltose binding protein (MBP) in its native state using continuous wave and double electron–electron resonance (DEER) electron paramagnetic resonance (EPR) spectroscopy. Seven double mutants had been employed to investigate the structure within the two domains of MBP. DEER data nicely corroborated the previously available X-ray data. Even in its MG state, MBP is known to still bind its ligand maltose. We therefore hypothesized that there must be a defined structure around the binding pocket of MBP already in the absence of tertiary structure. Here we have investigated the functional and structural difference between native and MG state in the open and closed form with a new set of MBP mutants. In these, the spin-label positions were placed near the active site. Binding of its ligands leads to a conformational change from open to closed state, where the two domains are more closely together. The complete set of MBP mutants was analyzed at pH 3.2 (MG) and pH 7.4 (native state) using double-quantum coherence EPR. The values were compared with theoretical predictions of distances between the labels in biradicals constructed by molecular modeling from the crystal structures of MBP in open and closed form and were found to be in excellent agreement. Measurements show a defined structure around the binding pocket of MBP in MG, which explains maltose binding. A new and important finding is that in both states ligand-free MBP can be found in open and closed form, while ligand-bound MBP appears only in closed form because of maltose binding.
中文翻译:

电子顺磁共振波谱研究天然状态和熔球状态下麦芽糖结合蛋白的开放和闭合形式
蛋白质折叠的一个深入研究的中间状态是熔球(MG)状态,它包含二级结构,但几乎没有三级结构。在之前的工作中,我们使用连续波和双电子-电子共振(DEER)电子顺磁共振(EPR)光谱确定了天然状态下麦芽糖结合蛋白(MBP)内相互作用自旋之间的距离。七个双突变体被用来研究 MBP 两个结构域内的结构。 DEER 数据很好地证实了之前可用的 X 射线数据。即使在 MG 状态下,MBP 仍与其配体麦芽糖结合。因此,我们假设在不存在三级结构的情况下,MBP 的结合口袋周围一定已经存在明确的结构。在这里,我们用一组新的 MBP 突变体研究了开放和封闭形式的天然状态和 MG 状态之间的功能和结构差异。在这些中,自旋标记位置被放置在活性位点附近。其配体的结合导致构象从开放状态变为闭合状态,其中两个结构域更加紧密地结合在一起。使用双量子相干 EPR 在 pH 3.2 (MG) 和 pH 7.4(天然状态)下分析整套 MBP 突变体。将这些值与根据开放和闭合形式的 MBP 晶体结构进行分子建模构建的双自由基中标记之间的距离的理论预测进行比较,结果发现非常一致。测量显示 MG 中 MBP 结合袋周围有明确的结构,这解释了麦芽糖结合。一项新的重要发现是,在这两种状态下,无配体的 MBP 均以开放和闭合形式存在,而配体结合的 MBP 由于麦芽糖结合而仅以闭合形式出现。
更新日期:2018-07-13
中文翻译:

电子顺磁共振波谱研究天然状态和熔球状态下麦芽糖结合蛋白的开放和闭合形式
蛋白质折叠的一个深入研究的中间状态是熔球(MG)状态,它包含二级结构,但几乎没有三级结构。在之前的工作中,我们使用连续波和双电子-电子共振(DEER)电子顺磁共振(EPR)光谱确定了天然状态下麦芽糖结合蛋白(MBP)内相互作用自旋之间的距离。七个双突变体被用来研究 MBP 两个结构域内的结构。 DEER 数据很好地证实了之前可用的 X 射线数据。即使在 MG 状态下,MBP 仍与其配体麦芽糖结合。因此,我们假设在不存在三级结构的情况下,MBP 的结合口袋周围一定已经存在明确的结构。在这里,我们用一组新的 MBP 突变体研究了开放和封闭形式的天然状态和 MG 状态之间的功能和结构差异。在这些中,自旋标记位置被放置在活性位点附近。其配体的结合导致构象从开放状态变为闭合状态,其中两个结构域更加紧密地结合在一起。使用双量子相干 EPR 在 pH 3.2 (MG) 和 pH 7.4(天然状态)下分析整套 MBP 突变体。将这些值与根据开放和闭合形式的 MBP 晶体结构进行分子建模构建的双自由基中标记之间的距离的理论预测进行比较,结果发现非常一致。测量显示 MG 中 MBP 结合袋周围有明确的结构,这解释了麦芽糖结合。一项新的重要发现是,在这两种状态下,无配体的 MBP 均以开放和闭合形式存在,而配体结合的 MBP 由于麦芽糖结合而仅以闭合形式出现。











































 京公网安备 11010802027423号
京公网安备 11010802027423号