当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photoaffinity Cross-Linking and Unnatural Amino Acid Mutagenesis Reveal Insights into Calcitonin Gene-Related Peptide Binding to the Calcitonin Receptor-like Receptor/Receptor Activity-Modifying Protein 1 (CLR/RAMP1) Complex
Biochemistry ( IF 2.9 ) Pub Date : 2018-07-13 00:00:00 , DOI: 10.1021/acs.biochem.8b00502 John Simms 1, 2 , Romez Uddin 1 , Thomas P Sakmar 3 , Joseph J Gingell 4 , Michael L Garelja 4 , Debbie L Hay 4 , Margaret A Brimble 4 , Paul W Harris 4 , Christopher A Reynolds 5 , David R Poyner 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-07-13 00:00:00 , DOI: 10.1021/acs.biochem.8b00502 John Simms 1, 2 , Romez Uddin 1 , Thomas P Sakmar 3 , Joseph J Gingell 4 , Michael L Garelja 4 , Debbie L Hay 4 , Margaret A Brimble 4 , Paul W Harris 4 , Christopher A Reynolds 5 , David R Poyner 1
Affiliation
|
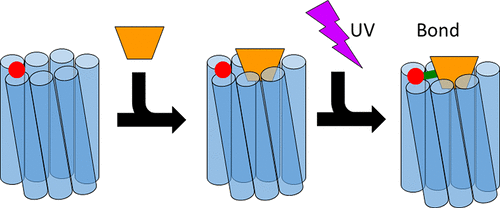
Calcitonin gene-related peptide (CGRP) binds to the complex of the calcitonin receptor-like receptor (CLR) with receptor activity-modifying protein 1 (RAMP1). How CGRP interacts with the transmembrane domain (including the extracellular loops) of this family B receptor remains unclear. In this study, a photoaffinity cross-linker, p-azido l-phenylalanine (azF), was incorporated into CLR, chiefly in the second extracellular loop (ECL2) using genetic code expansion and unnatural amino acid mutagenesis. The method was optimized to ensure efficient photolysis of azF residues near the transmembrane bundle of the receptor. A CGRP analogue modified with fluorescein at position 15 was used for detection of ultraviolet-induced cross-linking. The methodology was verified by confirming the known contacts of CGRP to the extracellular domain of CLR. Within ECL2, the chief contacts were I284 on the loop itself and L291, at the top of the fifth transmembrane helix (TM5). Minor contacts were noted along the lip of ECL2 between S286 and L290 and also with M223 in TM3 and F349 in TM6. Full length molecular models of the bound receptor complex suggest that CGRP sits at the top of the TM bundle, with Thr6 of the peptide making contacts with L291 and H295. I284 is likely to contact Leu12 and Ala13 of CGRP, and Leu16 of CGRP is at the ECL/extracellular domain boundary of CLR. The reduced potency, Emax, and affinity of [Leu16Ala]-human α CGRP are consistent with this model. Contacts between Thr6 of CGRP and H295 may be particularly important for receptor activation.
中文翻译:

光亲和交联和非天然氨基酸诱变揭示了降钙素基因相关肽与降钙素受体样受体/受体活性修饰蛋白 1 (CLR/RAMP1) 复合物结合的见解
降钙素基因相关肽 (CGRP) 与降钙素受体样受体 (CLR) 与受体活性修饰蛋白 1 (RAMP1) 的复合物结合。CGRP 如何与 B 家族受体的跨膜结构域(包括细胞外环)相互作用仍不清楚。在这项研究中,利用遗传密码扩展和非天然氨基酸诱变,将光亲和交联剂对叠氮基L-苯丙氨酸( azF) 纳入 CLR,主要是在第二个细胞外环 (ECL2) 中。该方法经过优化,确保受体跨膜束附近的 azF 残基有效光解。在第 15 位用荧光素修饰的 CGRP 类似物用于检测紫外线诱导的交联。通过确认 CGRP 与 CLR 胞外域的已知接触来验证该方法。在 ECL2 内,主要接触点是环本身上的 I284 和第五跨膜螺旋 (TM5) 顶部的 L291。注意到沿着S286和L290之间的ECL2边缘以及TM3中的M223和TM6中的F349之间存在少量接触。结合受体复合物的全长分子模型表明,CGRP 位于 TM 束的顶部,肽的Thr 6与 L291 和 H295 接触。I284可能与CGRP的Leu 12和Ala 13接触,并且CGRP的Leu 16位于CLR的ECL/胞外域边界。[Leu 16 Ala]-人 α CGRP 的降低效力、E max和亲和力与该模型一致。CGRP 的 Thr 6和 H295之间的接触对于受体激活可能特别重要。
更新日期:2018-07-13
中文翻译:

光亲和交联和非天然氨基酸诱变揭示了降钙素基因相关肽与降钙素受体样受体/受体活性修饰蛋白 1 (CLR/RAMP1) 复合物结合的见解
降钙素基因相关肽 (CGRP) 与降钙素受体样受体 (CLR) 与受体活性修饰蛋白 1 (RAMP1) 的复合物结合。CGRP 如何与 B 家族受体的跨膜结构域(包括细胞外环)相互作用仍不清楚。在这项研究中,利用遗传密码扩展和非天然氨基酸诱变,将光亲和交联剂对叠氮基L-苯丙氨酸( azF) 纳入 CLR,主要是在第二个细胞外环 (ECL2) 中。该方法经过优化,确保受体跨膜束附近的 azF 残基有效光解。在第 15 位用荧光素修饰的 CGRP 类似物用于检测紫外线诱导的交联。通过确认 CGRP 与 CLR 胞外域的已知接触来验证该方法。在 ECL2 内,主要接触点是环本身上的 I284 和第五跨膜螺旋 (TM5) 顶部的 L291。注意到沿着S286和L290之间的ECL2边缘以及TM3中的M223和TM6中的F349之间存在少量接触。结合受体复合物的全长分子模型表明,CGRP 位于 TM 束的顶部,肽的Thr 6与 L291 和 H295 接触。I284可能与CGRP的Leu 12和Ala 13接触,并且CGRP的Leu 16位于CLR的ECL/胞外域边界。[Leu 16 Ala]-人 α CGRP 的降低效力、E max和亲和力与该模型一致。CGRP 的 Thr 6和 H295之间的接触对于受体激活可能特别重要。



























 京公网安备 11010802027423号
京公网安备 11010802027423号