当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dynamic States of the Ligand-Free Class A G Protein-Coupled Receptor Extracellular Side
Biochemistry ( IF 2.9 ) Pub Date : 2018-07-12 00:00:00 , DOI: 10.1021/acs.biochem.8b00146 Qiansen Zhang 1 , Mang Zhou 2 , Lifen Zhao 2 , Hualiang Jiang 2 , Huaiyu Yang 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-07-12 00:00:00 , DOI: 10.1021/acs.biochem.8b00146 Qiansen Zhang 1 , Mang Zhou 2 , Lifen Zhao 2 , Hualiang Jiang 2 , Huaiyu Yang 1
Affiliation
|
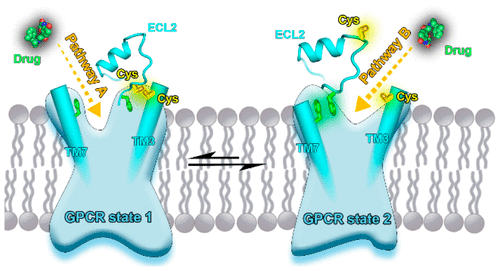
G protein-coupled receptors (GPCRs) make up the largest family of drug targets. The second extracellular loop (ECL2) and extracellular end of the third transmembrane helix (TM3) are basic structural elements of the GPCR ligand binding site. Currently, the disulfide bond between the two conserved cysteines in the ECL2 and TM3 is considered to be a basic GPCR structural feature. This disulfide bond has a significant effect on receptor dynamics and ligand binding. Here, molecular dynamics simulations and experimental results show that the two cysteines are distant from one another in the highest-population conformational state of ligand-free class A GPCRs and do not form a disulfide bond, indicating that the dynamics of the GPCR extracellular side are different from our conventional understanding. These surprising dynamics should have important effects on the drug binding process. On the basis of the two distinct ligand-free states, we suggest two kinetic processes for binding of ligands to GPCRs. These results challenge our commonly held beliefs regarding both GPCR structural features and ligand binding.
中文翻译:

无配体类AG蛋白偶联受体胞外侧的动态状态。
G蛋白偶联受体(GPCR)构成了最大的药物靶标家族。第二个细胞外环(ECL2)和第三个跨膜螺旋(TM3)的细胞外末端是GPCR配体结合位点的基本结构元件。目前,ECL2和TM3中两个保守半胱氨酸之间的二硫键被认为是基本的GPCR结构特征。该二硫键对受体动力学和配体结合具有显著作用。在这里,分子动力学模拟和实验结果表明,两个半胱氨酸在无配体的A类GPCR的最高种群构象状态中彼此远离,并且不形成二硫键,这表明GPCR细胞外一侧的动力学是与我们的传统理解不同。这些令人惊讶的动力学应该对药物结合过程产生重要影响。基于两个不同的无配体状态,我们提出了两个将配体与GPCR结合的动力学过程。这些结果挑战了我们关于GPCR结构特征和配体结合的普遍信念。
更新日期:2018-07-12
中文翻译:

无配体类AG蛋白偶联受体胞外侧的动态状态。
G蛋白偶联受体(GPCR)构成了最大的药物靶标家族。第二个细胞外环(ECL2)和第三个跨膜螺旋(TM3)的细胞外末端是GPCR配体结合位点的基本结构元件。目前,ECL2和TM3中两个保守半胱氨酸之间的二硫键被认为是基本的GPCR结构特征。该二硫键对受体动力学和配体结合具有显著作用。在这里,分子动力学模拟和实验结果表明,两个半胱氨酸在无配体的A类GPCR的最高种群构象状态中彼此远离,并且不形成二硫键,这表明GPCR细胞外一侧的动力学是与我们的传统理解不同。这些令人惊讶的动力学应该对药物结合过程产生重要影响。基于两个不同的无配体状态,我们提出了两个将配体与GPCR结合的动力学过程。这些结果挑战了我们关于GPCR结构特征和配体结合的普遍信念。









































 京公网安备 11010802027423号
京公网安备 11010802027423号