当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Steady-State Kinetics of Phytoglobin-Catalyzed Reduction of Hydroxylamine to Ammonium
Biochemistry ( IF 2.9 ) Pub Date : 2018-07-12 00:00:00 , DOI: 10.1021/acs.biochem.8b00586 Jagannathan Alagurajan 1 , Navjot Singh Athwal 1 , Mark S. Hargrove 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-07-12 00:00:00 , DOI: 10.1021/acs.biochem.8b00586 Jagannathan Alagurajan 1 , Navjot Singh Athwal 1 , Mark S. Hargrove 1
Affiliation
|
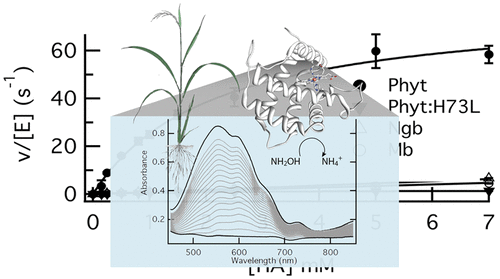
Phytoglobins are plant hexacoordinate hemoglobins with reversible coordination of a histidine side chain to the ligand binding site of the heme iron. They mediate electron transfer reactions such as nitric oxide scavenging and are particularly efficient at reducing nitrite and hydroxylamine. Previous work with phytoglobins has focused only on single turnovers of these reactions and has not revealed whether structural features, such as histidine hexacoordination, play a prominent role in the complete catalytic cycle. This work characterizes steady-state phytoglobin catalysis of reduction of hydroxylamine to ammonium using two different chemical reductants. Km and kcat values were measured for rice phytoglobin, horse myoglobin, human neuroglobin, and a rice phytoglobin mutant protein in which the hexacoordinating histidine has been replaced with leucine (Phyt:H73L). The results demonstrate that phytoglobin catalysis driven by benzyl viologen is limited only by the dissociation rate constant for the distal histidine. This is consistent with the rate limit in single-turnover experiments and demonstrates that the kinetics of hydroxylamine binding, and not phytoglobin reduction, ultimately governs the reaction. Catalysis by the other proteins that either lack or have tighter hexacoordination is much slower, suggesting that facile reversibility of the bond between the distal histidine and the heme iron is needed to allow both substrate binding and heme iron reduction. On the other hand, catalysis driven by dithionite is limited by SO2•– concentrations and is similar for all of these proteins, suggesting that dithionite is not a good reducing agent for evaluation of the catalytic properties of hemoglobins.
中文翻译:

珠蛋白催化羟胺还原成铵的稳态动力学
植物血红蛋白是具有组氨酸侧链与血红素铁的配体结合位点可逆配位的植物六配位血红蛋白。它们介导电子转移反应,例如清除一氧化氮,并且在还原亚硝酸盐和羟胺方面特别有效。以前对植物血红蛋白的研究仅集中于这些反应的一次转换,而没有揭示结构特征,例如组氨酸六配位在整个催化循环中是否起着重要作用。这项工作的特点是使用两种不同的化学还原剂对羟胺还原成铵的稳态植物血红蛋白催化。K m和k cat测量水稻植物红蛋白,马肌红蛋白,人神经球蛋白和水稻植物红蛋白突变蛋白的蛋白质值,其中六配位组氨酸已被亮氨酸替代(Phyt:H73L)。结果表明,由苄基紫精驱动的植物血红蛋白催化仅受远端组氨酸的解离速率常数的限制。这与单周转实验中的速率限制是一致的,并证明羟胺结合的动力学而不是植物血红蛋白的还原最终决定了反应。缺乏或具有更严格的六配位作用的其他蛋白质的催化作用要慢得多,这表明远端组氨酸和血红素铁之间键的容易可逆性需要底物结合和血红素铁还原。另一方面,2 •–浓度,并且对于所有这些蛋白质而言都是相似的,这表明连二亚硫酸盐不是评估血红蛋白催化特性的良好还原剂。
更新日期:2018-07-12
中文翻译:

珠蛋白催化羟胺还原成铵的稳态动力学
植物血红蛋白是具有组氨酸侧链与血红素铁的配体结合位点可逆配位的植物六配位血红蛋白。它们介导电子转移反应,例如清除一氧化氮,并且在还原亚硝酸盐和羟胺方面特别有效。以前对植物血红蛋白的研究仅集中于这些反应的一次转换,而没有揭示结构特征,例如组氨酸六配位在整个催化循环中是否起着重要作用。这项工作的特点是使用两种不同的化学还原剂对羟胺还原成铵的稳态植物血红蛋白催化。K m和k cat测量水稻植物红蛋白,马肌红蛋白,人神经球蛋白和水稻植物红蛋白突变蛋白的蛋白质值,其中六配位组氨酸已被亮氨酸替代(Phyt:H73L)。结果表明,由苄基紫精驱动的植物血红蛋白催化仅受远端组氨酸的解离速率常数的限制。这与单周转实验中的速率限制是一致的,并证明羟胺结合的动力学而不是植物血红蛋白的还原最终决定了反应。缺乏或具有更严格的六配位作用的其他蛋白质的催化作用要慢得多,这表明远端组氨酸和血红素铁之间键的容易可逆性需要底物结合和血红素铁还原。另一方面,2 •–浓度,并且对于所有这些蛋白质而言都是相似的,这表明连二亚硫酸盐不是评估血红蛋白催化特性的良好还原剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号