Synthesis ( IF 2.2 ) Pub Date : 2018-07-12 , DOI: 10.1055/s-0036-1591595 Philippe Compain 1 , Damien Tardieu 1 , Maria Céspedes Dávila 1 , Damien Hazelard 1
|
Abstract
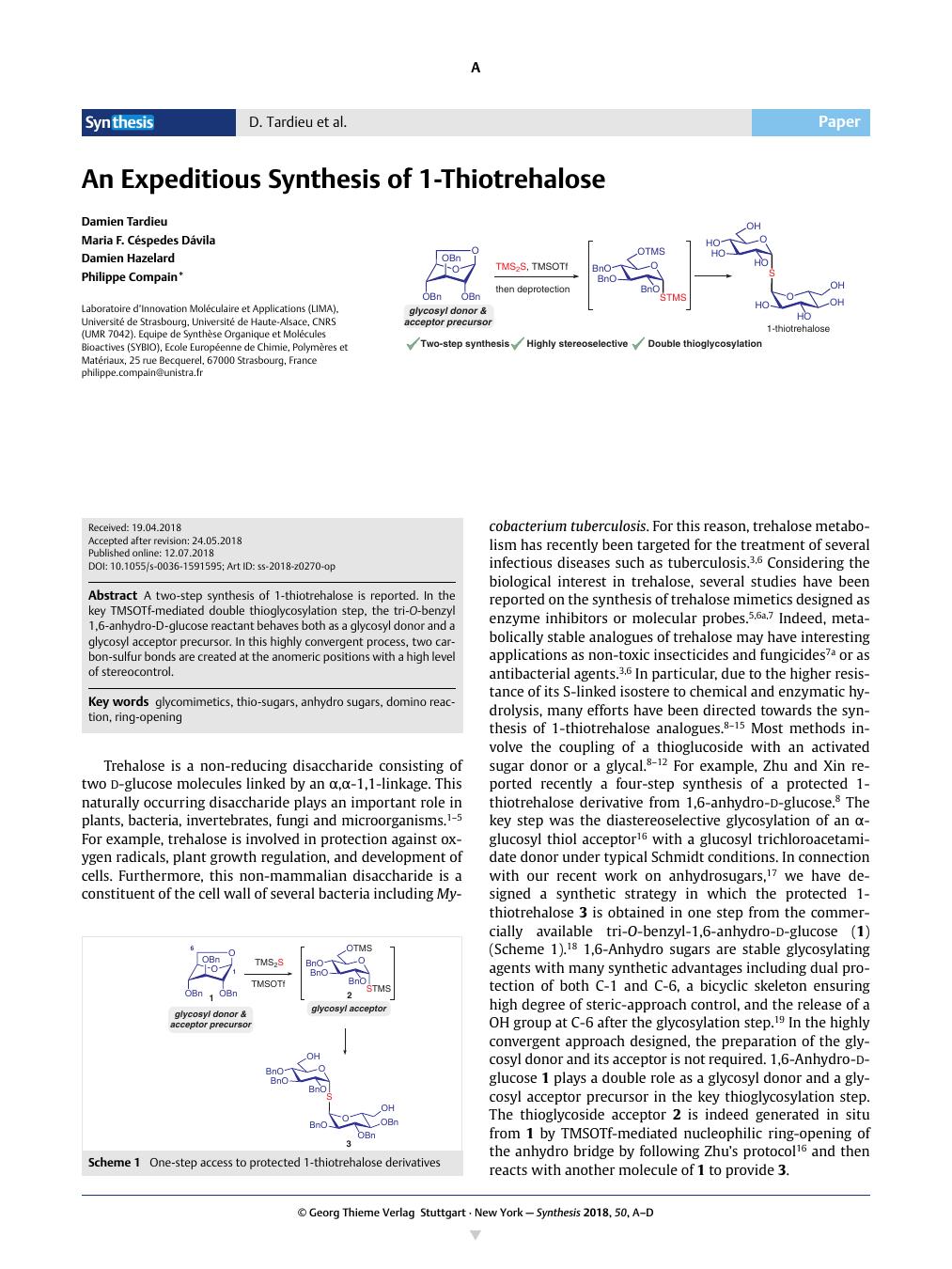
A two-step synthesis of 1-thiotrehalose is reported. In the key TMSOTf-mediated double thioglycosylation step, the tri-O-benzyl 1,6-anhydro-D-glucose reactant behaves both as a glycosyl donor and a glycosyl acceptor precursor. In this highly convergent process, two carbon-sulfur bonds are created at the anomeric positions with a high level of stereocontrol.
A two-step synthesis of 1-thiotrehalose is reported. In the key TMSOTf-mediated double thioglycosylation step, the tri-O-benzyl 1,6-anhydro-D-glucose reactant behaves both as a glycosyl donor and a glycosyl acceptor precursor. In this highly convergent process, two carbon-sulfur bonds are created at the anomeric positions with a high level of stereocontrol.
中文翻译:

1-硫代海藻糖的快速合成
摘要
报道了1-硫代海藻糖的两步合成。在关键的TMSOTf介导的双硫代糖基化步骤中,三-O-苄基1,6-脱水-D-葡萄糖反应物既充当糖基供体又充当糖基受体前体。在这种高度收敛的过程中,在具有高度立体控制水平的异头位置上产生了两个碳-硫键。
报道了1-硫代海藻糖的两步合成。在关键的TMSOTf介导的双硫代糖基化步骤中,三-O-苄基1,6-脱水-D-葡萄糖反应物既充当糖基供体又充当糖基受体前体。在这种高度收敛的过程中,在具有高度立体控制水平的异头位置上产生了两个碳-硫键。











































 京公网安备 11010802027423号
京公网安备 11010802027423号