当前位置:
X-MOL 学术
›
Cell Chem. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Meisenheimer Complex as a Paradigm in Drug Discovery: Reversible Covalent Inhibition through C67 of the ATP Binding Site of PLK1
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2018-07-12 , DOI: 10.1016/j.chembiol.2018.06.001 Russell J Pearson 1 , David G Blake 2 , Mokdad Mezna 2 , Peter M Fischer 3 , Nicholas J Westwood 4 , Campbell McInnes 5
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2018-07-12 , DOI: 10.1016/j.chembiol.2018.06.001 Russell J Pearson 1 , David G Blake 2 , Mokdad Mezna 2 , Peter M Fischer 3 , Nicholas J Westwood 4 , Campbell McInnes 5
Affiliation
|
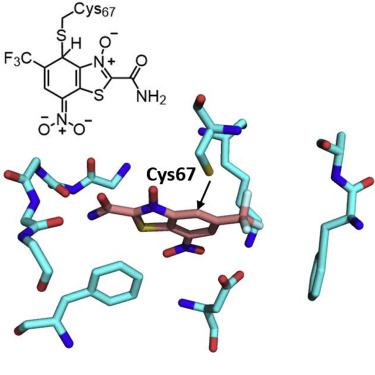
The polo kinase family are important oncology targets that act in regulating entry into and progression through mitosis. Structure-guided discovery of a new class of inhibitors of Polo-like kinase 1 (PLK1) catalytic activity that interact with Cys67 of the ATP binding site is described. Compounds containing the benzothiazoleN-oxide scaffold not only bind covalently to this residue, but are reversible inhibitors through the formation of Meisenheimer complexes. This mechanism of kinase inhibition results in compounds that can target PLK1 with high selectivity, while avoiding issues with irreversible covalent binding and interaction with other thiol-containing molecules in the cell. Due to renewed interest in covalent drugs and the plethora of potential drug targets, these represent prototypes for the design of kinase inhibitory compounds that achieve high specificity through covalent interaction and yet still bind reversibly to the ATP cleft, a strategy that could be applied to avoid issues with conventional covalent binders.
中文翻译:

Meisenheimer 复合物作为药物发现的范例:通过 PLK1 ATP 结合位点的 C67 进行可逆共价抑制
polo 激酶家族是重要的肿瘤学靶点,可调节有丝分裂的进入和进展。描述了以结构为指导发现的一类新型 Polo 样激酶 1 (PLK1) 催化活性抑制剂,该抑制剂可与 ATP 结合位点的 Cys67 相互作用。含有苯并噻唑N-氧化物支架的化合物不仅与该残基共价结合,而且通过形成 Meisenheimer 复合物成为可逆抑制剂。这种激酶抑制机制导致化合物能够高选择性地靶向 PLK1,同时避免与细胞中其他含硫醇分子不可逆共价结合和相互作用的问题。由于人们对共价药物和大量潜在药物靶标重新产生了兴趣,这些代表了激酶抑制化合物设计的原型,这些化合物通过共价相互作用实现了高特异性,但仍然可逆地结合到 ATP 裂隙上,这种策略可用于避免传统共价结合剂的问题。
更新日期:2018-09-20
中文翻译:

Meisenheimer 复合物作为药物发现的范例:通过 PLK1 ATP 结合位点的 C67 进行可逆共价抑制
polo 激酶家族是重要的肿瘤学靶点,可调节有丝分裂的进入和进展。描述了以结构为指导发现的一类新型 Polo 样激酶 1 (PLK1) 催化活性抑制剂,该抑制剂可与 ATP 结合位点的 Cys67 相互作用。含有苯并噻唑N-氧化物支架的化合物不仅与该残基共价结合,而且通过形成 Meisenheimer 复合物成为可逆抑制剂。这种激酶抑制机制导致化合物能够高选择性地靶向 PLK1,同时避免与细胞中其他含硫醇分子不可逆共价结合和相互作用的问题。由于人们对共价药物和大量潜在药物靶标重新产生了兴趣,这些代表了激酶抑制化合物设计的原型,这些化合物通过共价相互作用实现了高特异性,但仍然可逆地结合到 ATP 裂隙上,这种策略可用于避免传统共价结合剂的问题。











































 京公网安备 11010802027423号
京公网安备 11010802027423号