当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly efficient Pb(ii) and Cu(ii) removal using hollow Fe3O4@PDA nanoparticles with excellent application capability and reusability†
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2018-07-10 00:00:00 , DOI: 10.1039/c8qi00541a Ning Wang 1, 2, 3, 4, 5 , Dongxu Yang 1, 2, 3, 4, 5 , Xiangxue Wang 4, 5, 6, 7, 8 , Shujun Yu 4, 5, 6, 7 , Hongqing Wang 1, 2, 3, 4 , Tao Wen 4, 5, 6, 7 , Gang Song 8, 9, 10, 11, 12 , Zhimin Yu 4, 13, 14, 15 , Xiangke Wang 4, 5, 6, 7, 13
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2018-07-10 00:00:00 , DOI: 10.1039/c8qi00541a Ning Wang 1, 2, 3, 4, 5 , Dongxu Yang 1, 2, 3, 4, 5 , Xiangxue Wang 4, 5, 6, 7, 8 , Shujun Yu 4, 5, 6, 7 , Hongqing Wang 1, 2, 3, 4 , Tao Wen 4, 5, 6, 7 , Gang Song 8, 9, 10, 11, 12 , Zhimin Yu 4, 13, 14, 15 , Xiangke Wang 4, 5, 6, 7, 13
Affiliation
|
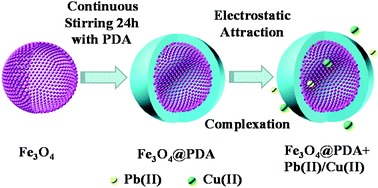
Potentially toxic metals in sewage and industrial effluents pose a serious threat to human health and the environment. Herein, core–shell hollow magnetic polydopamine nanoparticles (denoted as Fe3O4@PDA) were fabricated via a simple one-pot synthesis method and applied for the elimination of Pb(II) and Cu(II) from wastewater. The versatile polydopamine (PDA) layer with abundant functional groups (amine, imine and catechol groups) provided favourable sites to bind metal ions. The experimental results showed that the adsorption processes were significantly affected by the pH values of the suspension. The ionic strength-independent adsorption processes indicated the existence of inner-sphere surface complexes of Pb(II) and Cu(II) on Fe3O4@PDA. The results showed that Fe3O4@PDA exhibited fast removal kinetics for Pb(II) and Cu(II), which achieved equilibrium within 3 h. The adsorption processes were well fitted by the pseudo-second-order kinetic model and Langmuir isotherm model. Furthermore, the removal capacities of Fe3O4@PDA were calculated to be 57.25 mg g−1 for Pb(II) and 86.35 mg g−1 for Cu(II), which were much higher than those of pure Fe3O4 and other materials. Most importantly, the adsorption efficiencies of Pb(II) and Cu(II) on Fe3O4@PDA were still ∼71% and ∼70% after five cycles, respectively. In summary, the hollow Fe3O4@PDA nanoparticles can be used as outstanding materials for the elimination of Pb(II) and Cu(II), which is beneficial to reduce the toxic effects of heavy metal ion contaminated water.
中文翻译:

使用中空的Fe 3 O 4 @PDA纳米粒子 高效去除Pb(ii)和Cu(ii),具有出色的应用能力和可重复使用性†
污水和工业废水中的潜在有毒金属对人类健康和环境构成了严重威胁。在此,通过简单的一锅合成方法制备了核壳型空心磁性聚多巴胺纳米颗粒(称为Fe 3 O 4 @PDA),并用于消除Pb(II)和Cu(II))。具有丰富官能团(胺,亚胺和邻苯二酚基团)的通用聚多巴胺(PDA)层提供了结合金属离子的有利位置。实验结果表明,悬浮液的pH值对吸附过程有显着影响。离子强度无关的吸附过程表明在Fe 3 O 4 @PDA上存在Pb(II)和Cu(II)的内球表面复合物。结果表明,Fe 3 O 4 @PDA对Pb(II)和Cu(II)具有快速去除动力学。),在3小时内达到平衡。拟二级动力学模型和Langmuir等温线模型很好地拟合了吸附过程。此外,计算出的Fe 3 O 4 @PDA对Pb(II)的去除能力为57.25 mg g -1和对Cu(II)的86.35 mg g -1,远高于纯Fe 3 O 4的去除能力。和其他材料。最重要的是,经过五个循环后,Pb(II)和Cu(II)在Fe 3 O 4 @PDA上的吸附效率仍然分别为〜71%和〜70%。综上所述,空心Fe 3O 4 @PDA纳米颗粒可以用作消除Pb(II)和Cu(II)的杰出材料,这有利于减少重金属离子污染水的毒性。
更新日期:2018-07-10
中文翻译:

使用中空的Fe 3 O 4 @PDA纳米粒子 高效去除Pb(ii)和Cu(ii),具有出色的应用能力和可重复使用性†
污水和工业废水中的潜在有毒金属对人类健康和环境构成了严重威胁。在此,通过简单的一锅合成方法制备了核壳型空心磁性聚多巴胺纳米颗粒(称为Fe 3 O 4 @PDA),并用于消除Pb(II)和Cu(II))。具有丰富官能团(胺,亚胺和邻苯二酚基团)的通用聚多巴胺(PDA)层提供了结合金属离子的有利位置。实验结果表明,悬浮液的pH值对吸附过程有显着影响。离子强度无关的吸附过程表明在Fe 3 O 4 @PDA上存在Pb(II)和Cu(II)的内球表面复合物。结果表明,Fe 3 O 4 @PDA对Pb(II)和Cu(II)具有快速去除动力学。),在3小时内达到平衡。拟二级动力学模型和Langmuir等温线模型很好地拟合了吸附过程。此外,计算出的Fe 3 O 4 @PDA对Pb(II)的去除能力为57.25 mg g -1和对Cu(II)的86.35 mg g -1,远高于纯Fe 3 O 4的去除能力。和其他材料。最重要的是,经过五个循环后,Pb(II)和Cu(II)在Fe 3 O 4 @PDA上的吸附效率仍然分别为〜71%和〜70%。综上所述,空心Fe 3O 4 @PDA纳米颗粒可以用作消除Pb(II)和Cu(II)的杰出材料,这有利于减少重金属离子污染水的毒性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号