当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conformation-guided analogue design identifies potential antimalarial compounds through inhibition of mitochondrial respiration.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-07-11 00:00:00 , DOI: 10.1039/c8ob01257a Erik M Larsen 1 , Chia-Fu Chang , Tomoyo Sakata-Kato , Joseph W Arico , Vince M Lombardo , Dyann F Wirth , Richard E Taylor
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-07-11 00:00:00 , DOI: 10.1039/c8ob01257a Erik M Larsen 1 , Chia-Fu Chang , Tomoyo Sakata-Kato , Joseph W Arico , Vince M Lombardo , Dyann F Wirth , Richard E Taylor
Affiliation
|
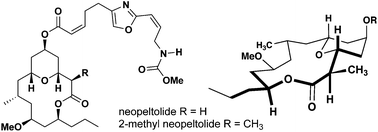
The synthesis of a 2-methyl-substituted analogue of the natural product, neopeltolide, is reported in an effort to analyze the importance of molecular conformation and ligand–target interactions in relation to biological activity. The methyl substitution was incorporated via highly diastereoselective ester enolate alkylation of a late-stage intermediate. Coupling of the oxazole sidechain provided 2-methyl-neopeltolide and synthetic neopeltolide via total synthesis. The substitution was shown to maintain the conformational preferences of its biologically active parent compound through computer modeling and NMR studies. Both compounds were shown to be potential antimalarial compounds through the inhibition of mitochondrial respiration in P. falciparum parasites.
中文翻译:

构象引导的类似物设计通过抑制线粒体呼吸来识别潜在的抗疟化合物。
据报道,天然产物 Neopeltolide 的 2-甲基取代类似物的合成是为了分析分子构象和配体-靶标相互作用对生物活性的重要性。通过后期中间体的高度非对映选择性酯烯醇化物烷基化引入甲基取代。恶唑侧链的偶联提供了 2-甲基-新佩尔内酯和通过全合成合成的新佩尔内酯。通过计算机建模和核磁共振研究表明,这种取代可以保持其生物活性母体化合物的构象偏好。通过抑制恶性疟原虫的线粒体呼吸,这两种化合物被证明是潜在的抗疟化合物。
更新日期:2018-07-11
中文翻译:

构象引导的类似物设计通过抑制线粒体呼吸来识别潜在的抗疟化合物。
据报道,天然产物 Neopeltolide 的 2-甲基取代类似物的合成是为了分析分子构象和配体-靶标相互作用对生物活性的重要性。通过后期中间体的高度非对映选择性酯烯醇化物烷基化引入甲基取代。恶唑侧链的偶联提供了 2-甲基-新佩尔内酯和通过全合成合成的新佩尔内酯。通过计算机建模和核磁共振研究表明,这种取代可以保持其生物活性母体化合物的构象偏好。通过抑制恶性疟原虫的线粒体呼吸,这两种化合物被证明是潜在的抗疟化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号