当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A theoretical study on NHC-catalysed enantioselective cycloaddition of ketenes and 3-aroylcoumarins: mechanism and enantioselectivity†
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-07-11 00:00:00 , DOI: 10.1039/c8ob01035h Ramón J. Zaragozá 1, 2, 3, 4 , María J. Aurell 1, 2, 3, 4 , Miguel A. González-Cardenete 4, 5, 6, 7
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-07-11 00:00:00 , DOI: 10.1039/c8ob01035h Ramón J. Zaragozá 1, 2, 3, 4 , María J. Aurell 1, 2, 3, 4 , Miguel A. González-Cardenete 4, 5, 6, 7
Affiliation
|
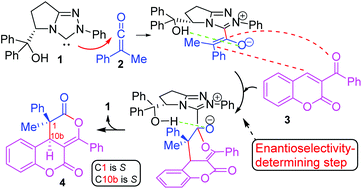
NHC-catalysed enantioselective cycloaddition of ketenes to 3-aroylcoumarins to yield dihydrocoumarin-fused dihydropyranones has been investigated using DFT methods at the B3LYP/6-31G* and MPWB1K/6-311G** computational levels. Two plausible mechanisms have been studied: the “ketene-first” mechanism A and the “coumarin-first” mechanism B. An analysis of the activation Gibbs free energies involved in the two competitive pathways makes it possible to rule out the pathway associated with the “coumarin-first” mechanism B. The first step of the “ketene-first” mechanism A is the formation of zwitterionic intermediate IN1-Zvia a nucleophilic attack of NHC 1 on ketene 2. A [4 + 2] cycloaddition through the nucleophilic attack of enolate IN1-Z on the conjugated double bond of the benzoyl group of coumarin 3, viaTS3-SS-a2 or TS3-RR-a2, yields IN3. Finally, the extrusion of the catalyst through TS5 leads to the final products, either 4-SS or 4-RR. Enantioselectivity observed in the experimental results is determined in the transition states TS3-SS-a2/TS3-RR-a2. In this pathway, the intramolecular hydrogen-bonding between the hydroxyl group of the IN1-Z adduct and the carbonyl oxygen of the original ketene group directs the final stereochemistry throughout the entire process.
中文翻译:

NHC催化烯酮和3-芳酰基香豆素的对映选择性环加成的理论研究:机理和对映选择性†
使用DFT方法在B3LYP / 6-31G *和MPWB1K / 6-311G **计算水平上研究了NHC催化的烯酮与3-芳酰基香豆素的对映选择性环加成反应生成二氢香豆素稠合的二氢吡喃酮。已研究了两种可能的机制:“烯酮优先”机制A和“香豆素优先”机制B。对两种竞争途径中涉及的激活吉布斯自由能的分析,有可能排除与该途径相关的途径。 “香豆素优先”机理B。“烯酮优先”机理A的第一步是通过NHC 1对烯酮2的亲核攻击形成两性离子中间体IN1- Z。通过烯醇盐的亲核攻击进行的[4 + 2]环加成反应IN1- ž对苯甲酰基的香豆素的共轭双键3,经由TS3- SS -a2或TS3- RR -a2,产生IN3。最后,通过TS5挤出催化剂得到最终产物4- SS或4- RR。实验结果中观察到的对映选择性是在过渡态TS3- SS -a2 / TS3- RR -a2中确定的。在此途径中,IN1-的羟基之间的分子内氢键Z加合物和原始烯酮基团的羰基氧在整个过程中指导最终的立体化学。
更新日期:2018-07-11
中文翻译:

NHC催化烯酮和3-芳酰基香豆素的对映选择性环加成的理论研究:机理和对映选择性†
使用DFT方法在B3LYP / 6-31G *和MPWB1K / 6-311G **计算水平上研究了NHC催化的烯酮与3-芳酰基香豆素的对映选择性环加成反应生成二氢香豆素稠合的二氢吡喃酮。已研究了两种可能的机制:“烯酮优先”机制A和“香豆素优先”机制B。对两种竞争途径中涉及的激活吉布斯自由能的分析,有可能排除与该途径相关的途径。 “香豆素优先”机理B。“烯酮优先”机理A的第一步是通过NHC 1对烯酮2的亲核攻击形成两性离子中间体IN1- Z。通过烯醇盐的亲核攻击进行的[4 + 2]环加成反应IN1- ž对苯甲酰基的香豆素的共轭双键3,经由TS3- SS -a2或TS3- RR -a2,产生IN3。最后,通过TS5挤出催化剂得到最终产物4- SS或4- RR。实验结果中观察到的对映选择性是在过渡态TS3- SS -a2 / TS3- RR -a2中确定的。在此途径中,IN1-的羟基之间的分子内氢键Z加合物和原始烯酮基团的羰基氧在整个过程中指导最终的立体化学。



























 京公网安备 11010802027423号
京公网安备 11010802027423号